Benjamin E. Nye
Understanding Clinical Trial Reports: Extracting Medical Entities and Their Relations
Oct 08, 2020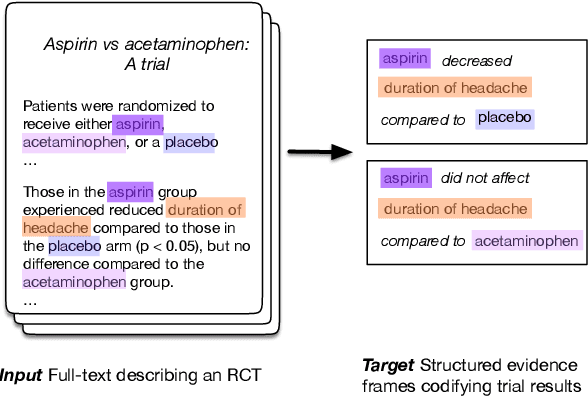
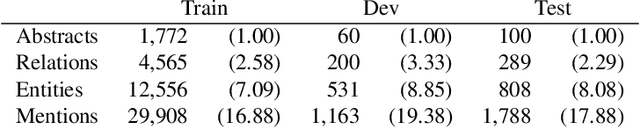
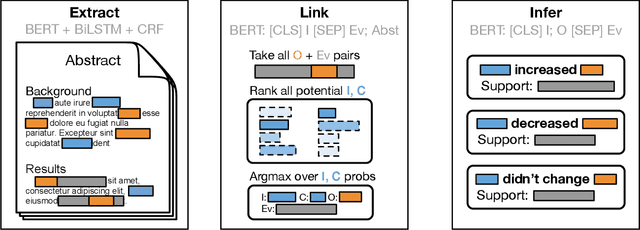

Abstract:The best evidence concerning comparative treatment effectiveness comes from clinical trials, the results of which are reported in unstructured articles. Medical experts must manually extract information from articles to inform decision-making, which is time-consuming and expensive. Here we consider the end-to-end task of both (a) extracting treatments and outcomes from full-text articles describing clinical trials (entity identification) and, (b) inferring the reported results for the former with respect to the latter (relation extraction). We introduce new data for this task, and evaluate models that have recently achieved state-of-the-art results on similar tasks in Natural Language Processing. We then propose a new method motivated by how trial results are typically presented that outperforms these purely data-driven baselines. Finally, we run a fielded evaluation of the model with a non-profit seeking to identify existing drugs that might be re-purposed for cancer, showing the potential utility of end-to-end evidence extraction systems.
Trialstreamer: Mapping and Browsing Medical Evidence in Real-Time
May 21, 2020



Abstract:We introduce Trialstreamer, a living database of clinical trial reports. Here we mainly describe the evidence extraction component; this extracts from biomedical abstracts key pieces of information that clinicians need when appraising the literature, and also the relations between these. Specifically, the system extracts descriptions of trial participants, the treatments compared in each arm (the interventions), and which outcomes were measured. The system then attempts to infer which interventions were reported to work best by determining their relationship with identified trial outcome measures. In addition to summarizing individual trials, these extracted data elements allow automatic synthesis of results across many trials on the same topic. We apply the system at scale to all reports of randomized controlled trials indexed in MEDLINE, powering the automatic generation of evidence maps, which provide a global view of the efficacy of different interventions combining data from all relevant clinical trials on a topic. We make all code and models freely available alongside a demonstration of the web interface.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge