Alice Del Vecchio
Season combinatorial intervention predictions with Salt & Peper
Apr 25, 2024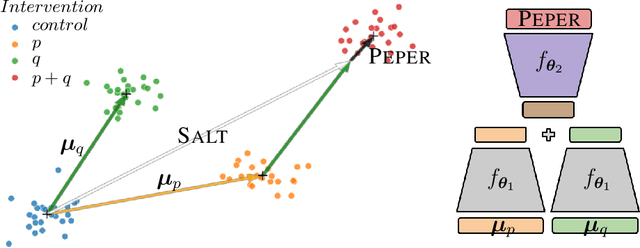
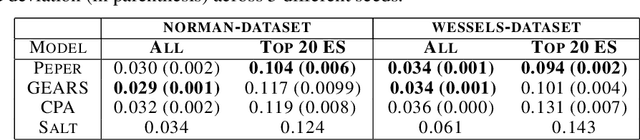
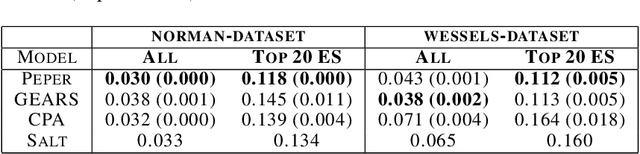
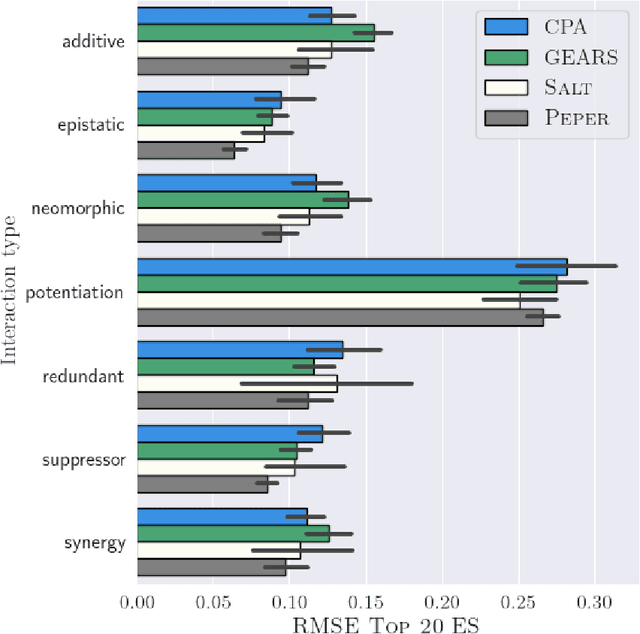
Abstract:Interventions play a pivotal role in the study of complex biological systems. In drug discovery, genetic interventions (such as CRISPR base editing) have become central to both identifying potential therapeutic targets and understanding a drug's mechanism of action. With the advancement of CRISPR and the proliferation of genome-scale analyses such as transcriptomics, a new challenge is to navigate the vast combinatorial space of concurrent genetic interventions. Addressing this, our work concentrates on estimating the effects of pairwise genetic combinations on the cellular transcriptome. We introduce two novel contributions: Salt, a biologically-inspired baseline that posits the mostly additive nature of combination effects, and Peper, a deep learning model that extends Salt's additive assumption to achieve unprecedented accuracy. Our comprehensive comparison against existing state-of-the-art methods, grounded in diverse metrics, and our out-of-distribution analysis highlight the limitations of current models in realistic settings. This analysis underscores the necessity for improved modelling techniques and data acquisition strategies, paving the way for more effective exploration of genetic intervention effects.
Neural message passing for joint paratope-epitope prediction
May 31, 2021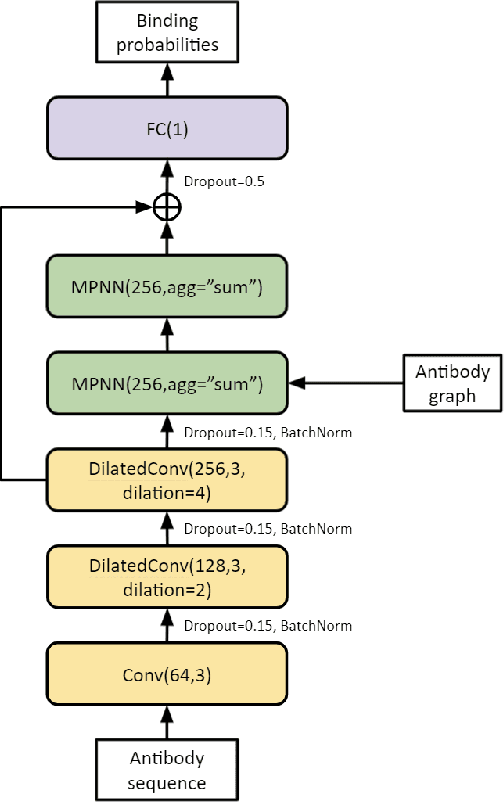
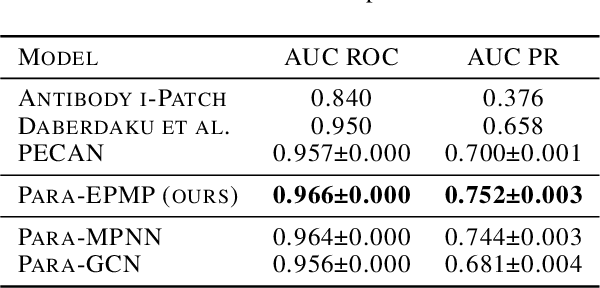
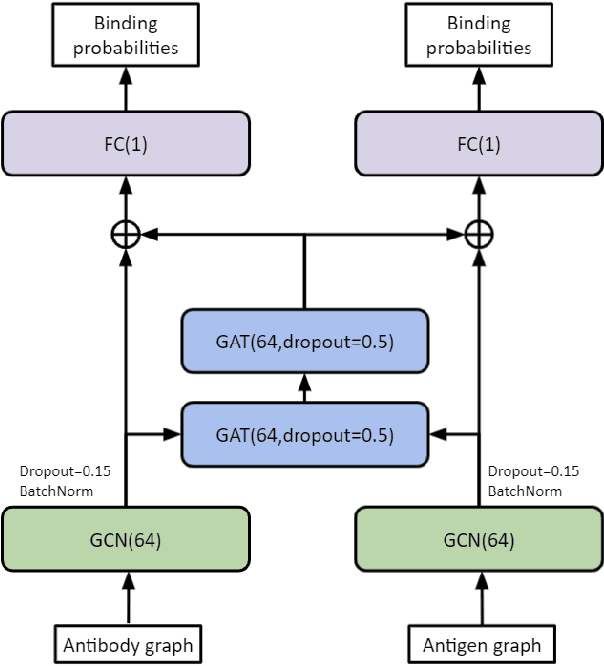
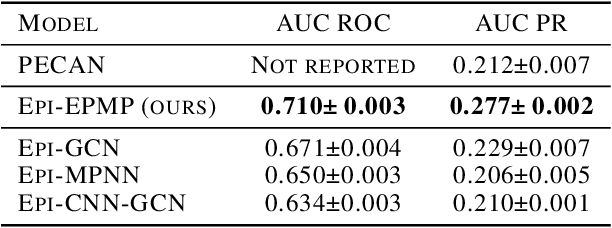
Abstract:Antibodies are proteins in the immune system which bind to antigens to detect and neutralise them. The binding sites in an antibody-antigen interaction are known as the paratope and epitope, respectively, and the prediction of these regions is key to vaccine and synthetic antibody development. Contrary to prior art, we argue that paratope and epitope predictors require asymmetric treatment, and propose distinct neural message passing architectures that are geared towards the specific aspects of paratope and epitope prediction, respectively. We obtain significant improvements on both tasks, setting the new state-of-the-art and recovering favourable qualitative predictions on antigens of relevance to COVID-19.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge