Abramo Agosti
Deep Anatomical Federated Network (Dafne): an open client/server framework for the continuous collaborative improvement of deep-learning-based medical image segmentation
Feb 14, 2023Abstract:Semantic segmentation is a crucial step to extract quantitative information from medical (and, specifically, radiological) images to aid the diagnostic process, clinical follow-up. and to generate biomarkers for clinical research. In recent years, machine learning algorithms have become the primary tool for this task. However, its real-world performance is heavily reliant on the comprehensiveness of training data. Dafne is the first decentralized, collaborative solution that implements continuously evolving deep learning models exploiting the collective knowledge of the users of the system. In the Dafne workflow, the result of each automated segmentation is refined by the user through an integrated interface, so that the new information is used to continuously expand the training pool via federated incremental learning. The models deployed through Dafne are able to improve their performance over time and to generalize to data types not seen in the training sets, thus becoming a viable and practical solution for real-life medical segmentation tasks.
Quantification of pulmonary involvement in COVID-19 pneumonia by means of a cascade oftwo U-nets: training and assessment on multipledatasets using different annotation criteria
May 06, 2021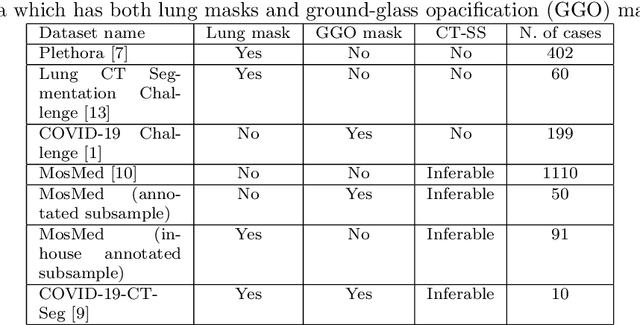
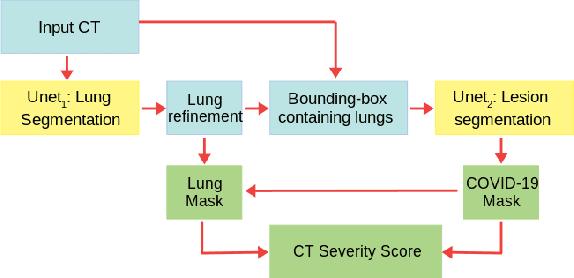
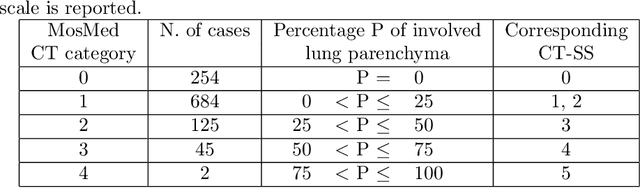
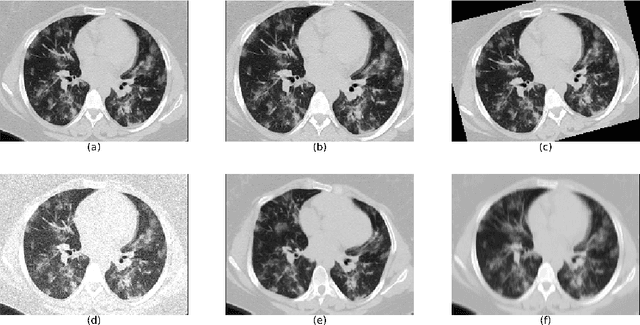
Abstract:The automatic assignment of a severity score to the CT scans of patients affected by COVID-19 pneumonia could reduce the workload in radiology departments. This study aims at exploiting Artificial intelligence (AI) for the identification, segmentation and quantification of COVID-19 pulmonary lesions. We investigated the effects of using multiple datasets, heterogeneously populated and annotated according to different criteria. We developed an automated analysis pipeline, the LungQuant system, based on a cascade of two U-nets. The first one (U-net_1) is devoted to the identification of the lung parenchyma, the second one (U-net_2) acts on a bounding box enclosing the segmented lungs to identify the areas affected by COVID-19 lesions. Different public datasets were used to train the U-nets and to evaluate their segmentation performances, which have been quantified in terms of the Dice index. The accuracy in predicting the CT-Severity Score (CT-SS) of the LungQuant system has been also evaluated. Both Dice and accuracy showed a dependency on the quality of annotations of the available data samples. On an independent and publicly available benchmark dataset, the Dice values measured between the masks predicted by LungQuant system and the reference ones were 0.95$\pm$0.01 and 0.66$\pm$0.13 for the segmentation of lungs and COVID-19 lesions, respectively. The accuracy of 90% in the identification of the CT-SS on this benchmark dataset was achieved. We analysed the impact of using data samples with different annotation criteria in training an AI-based quantification system for pulmonary involvement in COVID-19 pneumonia. In terms of the Dice index, the U-net segmentation quality strongly depends on the quality of the lesion annotations. Nevertheless, the CT-SS can be accurately predicted on independent validation sets, demonstrating the satisfactory generalization ability of the LungQuant.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge