Aart J. Nederveen
ASL to PET Translation by a Semi-supervised Residual-based Attention-guided Convolutional Neural Network
Mar 08, 2021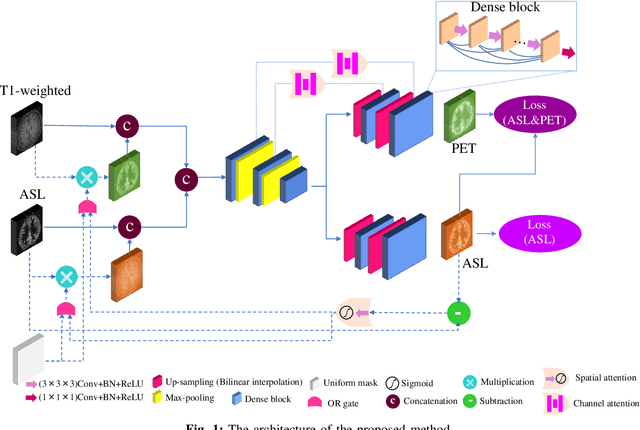
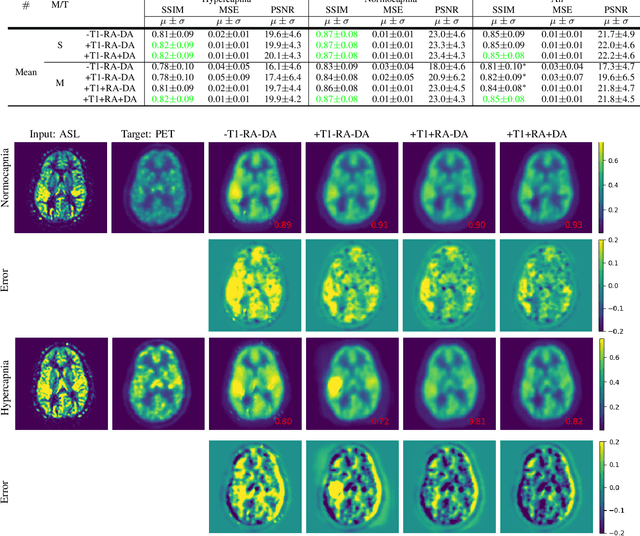
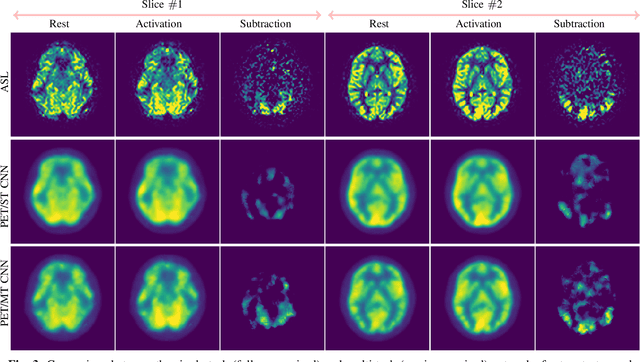
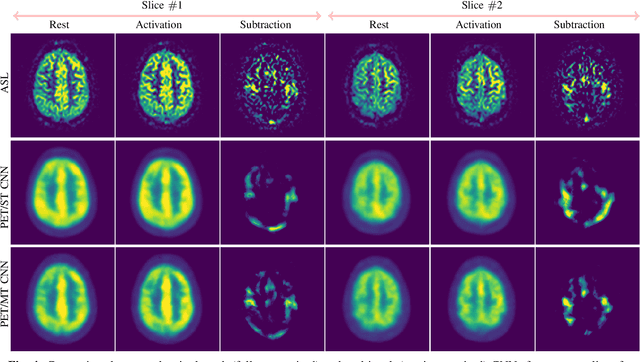
Abstract:Positron Emission Tomography (PET) is an imaging method that can assess physiological function rather than structural disturbances by measuring cerebral perfusion or glucose consumption. However, this imaging technique relies on injection of radioactive tracers and is expensive. On the contrary, Arterial Spin Labeling (ASL) MRI is a non-invasive, non-radioactive, and relatively cheap imaging technique for brain hemodynamic measurements, which allows quantification to some extent. In this paper we propose a convolutional neural network (CNN) based model for translating ASL to PET images, which could benefit patients as well as the healthcare system in terms of expenses and adverse side effects. However, acquiring a sufficient number of paired ASL-PET scans for training a CNN is prohibitive for many reasons. To tackle this problem, we present a new semi-supervised multitask CNN which is trained on both paired data, i.e. ASL and PET scans, and unpaired data, i.e. only ASL scans, which alleviates the problem of training a network on limited paired data. Moreover, we present a new residual-based-attention guided mechanism to improve the contextual features during the training process. Also, we show that incorporating T1-weighted scans as an input, due to its high resolution and availability of anatomical information, improves the results. We performed a two-stage evaluation based on quantitative image metrics by conducting a 7-fold cross validation followed by a double-blind observer study. The proposed network achieved structural similarity index measure (SSIM), mean squared error (MSE) and peak signal-to-noise ratio (PSNR) values of $0.85\pm0.08$, $0.01\pm0.01$, and $21.8\pm4.5$ respectively, for translating from 2D ASL and T1-weighted images to PET data. The proposed model is publicly available via https://github.com/yousefis/ASL2PET.
Improved unsupervised physics-informed deep learning for intravoxel-incoherent motion modeling and evaluation in pancreatic cancer patients
Nov 03, 2020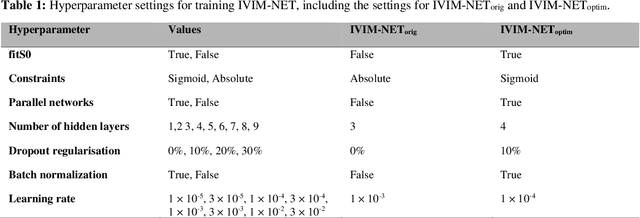
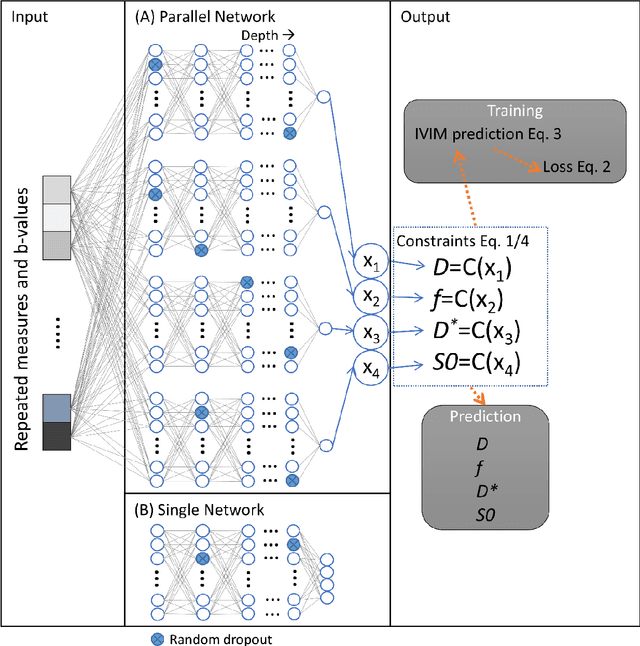
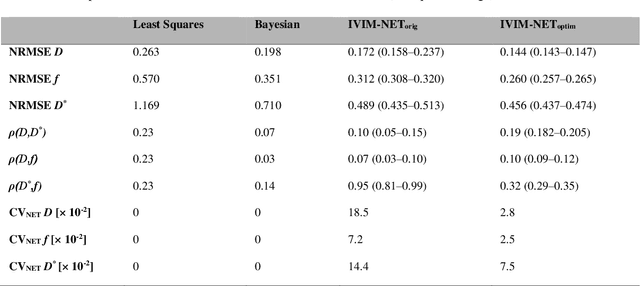
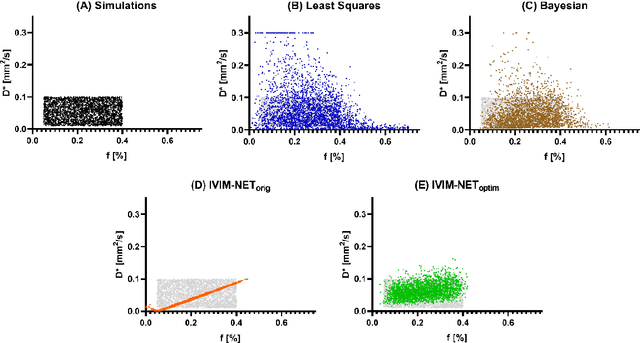
Abstract:${\bf Purpose}$: Earlier work showed that IVIM-NET$_{orig}$, an unsupervised physics-informed deep neural network, was faster and more accurate than other state-of-the-art intravoxel-incoherent motion (IVIM) fitting approaches to DWI. This study presents: IVIM-NET$_{optim}$, overcoming IVIM-NET$_{orig}$'s shortcomings. ${\bf Method}$: In simulations (SNR=20), the accuracy, independence and consistency of IVIM-NET were evaluated for combinations of hyperparameters (fit S0, constraints, network architecture, # hidden layers, dropout, batch normalization, learning rate), by calculating the NRMSE, Spearman's $\rho$, and the coefficient of variation (CV$_{NET}$), respectively. The best performing network, IVIM-NET$_{optim}$ was compared to least squares (LS) and a Bayesian approach at different SNRs. IVIM-NET$_{optim}$'s performance was evaluated in 23 pancreatic ductal adenocarcinoma (PDAC) patients. 14 of the patients received no treatment between 2 repeated scan sessions and 9 received chemoradiotherapy between sessions. Intersession within-subject standard deviations (wSD) and treatment-induced changes were assessed. ${\bf Results}$: In simulations, IVIM-NET$_{optim}$ outperformed IVIM-NET$_{orig}$ in accuracy (NRMSE(D)=0.14 vs 0.17; NMRSE(f)=0.26 vs 0.31; NMRSE(D*)=0.46 vs 0.49), independence ($\rho$(D*,f)=0.32 vs 0.95) and consistency (CV$_{NET}$ (D)=0.028 vs 0.185; CV$_{NET}$ (f)=0.025 vs 0.078; CV$_{NET}$ (D*)=0.075 vs 0.144). IVIM-NET$_{optim}$ showed superior performance to the LS and Bayesian approaches at SNRs<50. In vivo, IVIM-NET$_{optim}$ showed less noisy and more detailed parameter maps with lower wSD for D and f than the alternatives. In the treated cohort, IVIM-NET$_{optim}$ detected the most individual patients with significant parameter changes compared to day-to-day variations. ${\bf Conclusion}$: IVIM-NET$_{optim}$ is recommended for accurate IVIM fitting to DWI data.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge