The Whole Pathological Slide Classification via Weakly Supervised Learning
Paper and Code
Jul 12, 2023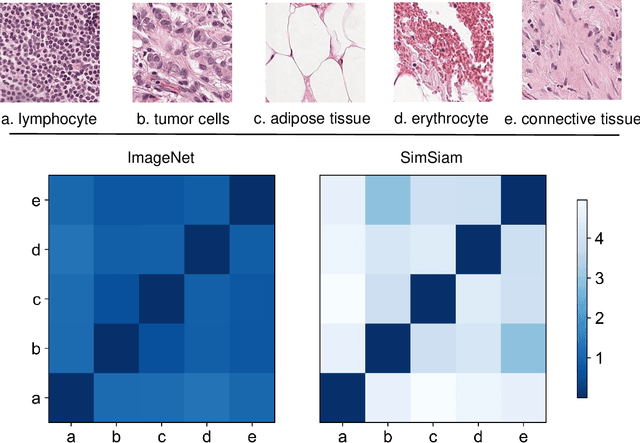
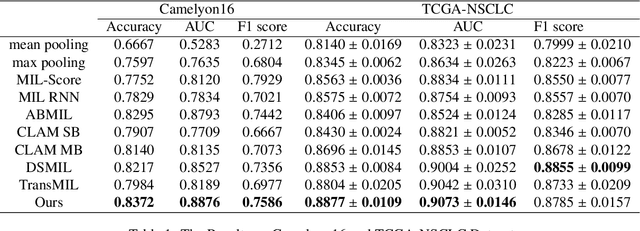
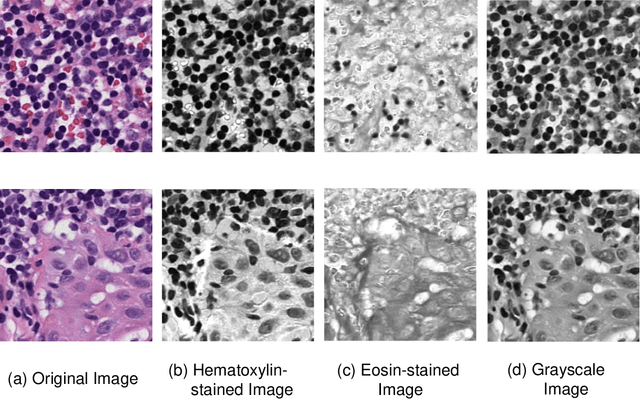

Due to its superior efficiency in utilizing annotations and addressing gigapixel-sized images, multiple instance learning (MIL) has shown great promise as a framework for whole slide image (WSI) classification in digital pathology diagnosis. However, existing methods tend to focus on advanced aggregators with different structures, often overlooking the intrinsic features of H\&E pathological slides. To address this limitation, we introduced two pathological priors: nuclear heterogeneity of diseased cells and spatial correlation of pathological tiles. Leveraging the former, we proposed a data augmentation method that utilizes stain separation during extractor training via a contrastive learning strategy to obtain instance-level representations. We then described the spatial relationships between the tiles using an adjacency matrix. By integrating these two views, we designed a multi-instance framework for analyzing H\&E-stained tissue images based on pathological inductive bias, encompassing feature extraction, filtering, and aggregation. Extensive experiments on the Camelyon16 breast dataset and TCGA-NSCLC Lung dataset demonstrate that our proposed framework can effectively handle tasks related to cancer detection and differentiation of subtypes, outperforming state-of-the-art medical image classification methods based on MIL. The code will be released later.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge