Signalling Paediatric Side Effects using an Ensemble of Simple Study Designs
Paper and Code
Sep 02, 2014
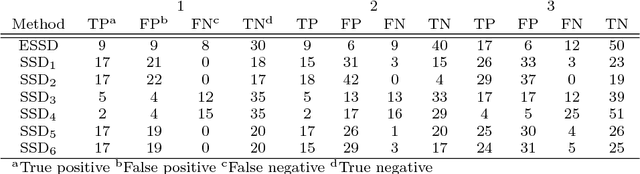
Background: Children are frequently prescribed medication off-label, meaning there has not been sufficient testing of the medication to determine its safety or effectiveness. The main reason this safety knowledge is lacking is due to ethical restrictions that prevent children from being included in the majority of clinical trials. Objective: The objective of this paper is to investigate whether an ensemble of simple study designs can be implemented to signal acutely occurring side effects effectively within the paediatric population by using historical longitudinal data. The majority of pharmacovigilance techniques are unsupervised, but this research presents a supervised framework. Methods: Multiple measures of association are calculated for each drug and medical event pair and these are used as features that are fed into a classiffier to determine the likelihood of the drug and medical event pair corresponding to an adverse drug reaction. The classiffier is trained using known adverse drug reactions or known non-adverse drug reaction relationships. Results: The novel ensemble framework obtained a false positive rate of 0:149, a sensitivity of 0:547 and a specificity of 0:851 when implemented on a reference set of drug and medical event pairs. The novel framework consistently outperformed each individual simple study design. Conclusion: This research shows that it is possible to exploit the mechanism of causality and presents a framework for signalling adverse drug reactions effectively.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge