SDF-Bayes: Cautious Optimism in Safe Dose-Finding Clinical Trials with Drug Combinations and Heterogeneous Patient Groups
Paper and Code
Jan 26, 2021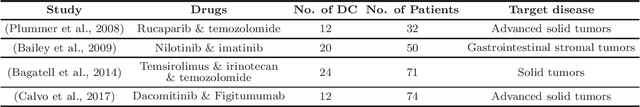
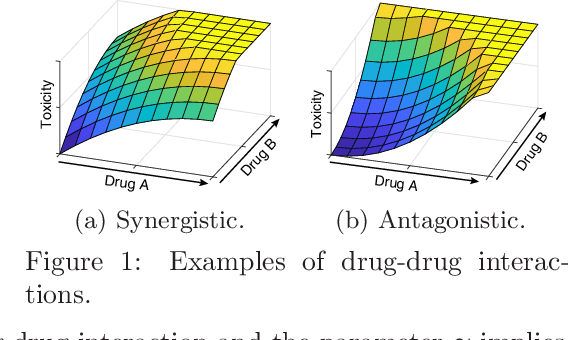

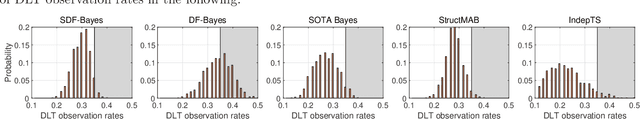
Phase I clinical trials are designed to test the safety (non-toxicity) of drugs and find the maximum tolerated dose (MTD). This task becomes significantly more challenging when multiple-drug dose-combinations (DC) are involved, due to the inherent conflict between the exponentially increasing DC candidates and the limited patient budget. This paper proposes a novel Bayesian design, SDF-Bayes, for finding the MTD for drug combinations in the presence of safety constraints. Rather than the conventional principle of escalating or de-escalating the current dose of one drug (perhaps alternating between drugs), SDF-Bayes proceeds by cautious optimism: it chooses the next DC that, on the basis of current information, is most likely to be the MTD (optimism), subject to the constraint that it only chooses DCs that have a high probability of being safe (caution). We also propose an extension, SDF-Bayes-AR, that accounts for patient heterogeneity and enables heterogeneous patient recruitment. Extensive experiments based on both synthetic and real-world datasets demonstrate the advantages of SDF-Bayes over state of the art DC trial designs in terms of accuracy and safety.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge