Zhifang Wu
Clinically Translatable Direct Patlak Reconstruction from Dynamic PET with Motion Correction Using Convolutional Neural Network
Sep 13, 2020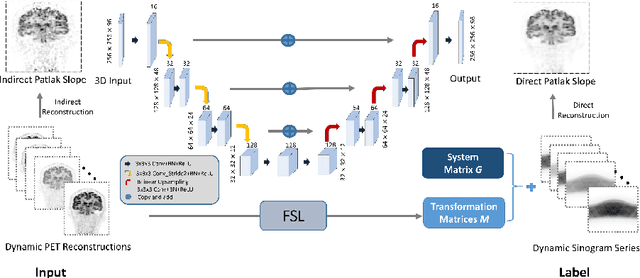

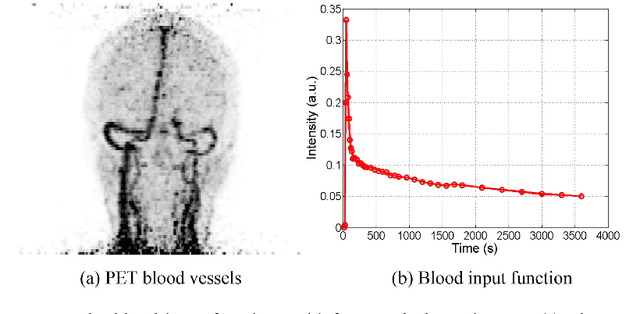
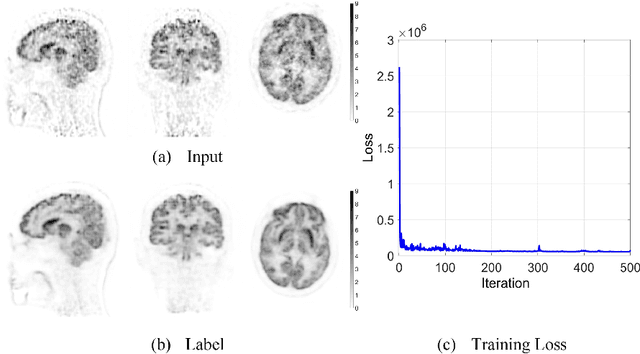
Abstract:Patlak model is widely used in 18F-FDG dynamic positron emission tomography (PET) imaging, where the estimated parametric images reveal important biochemical and physiology information. Because of better noise modeling and more information extracted from raw sinogram, direct Patlak reconstruction gains its popularity over the indirect approach which utilizes reconstructed dynamic PET images alone. As the prerequisite of direct Patlak methods, raw data from dynamic PET are rarely stored in clinics and difficult to obtain. In addition, the direct reconstruction is time-consuming due to the bottleneck of multiple-frame reconstruction. All of these impede the clinical adoption of direct Patlak reconstruction.In this work, we proposed a data-driven framework which maps the dynamic PET images to the high-quality motion-corrected direct Patlak images through a convolutional neural network. For the patient motion during the long period of dynamic PET scan, we combined the correction with the backward/forward projection in direct reconstruction to better fit the statistical model. Results based on fifteen clinical 18F-FDG dynamic brain PET datasets demonstrates the superiority of the proposed framework over Gaussian, nonlocal mean and BM4D denoising, regarding the image bias and contrast-to-noise ratio.
Penalized-likelihood PET Image Reconstruction Using 3D Structural Convolutional Sparse Coding
Dec 16, 2019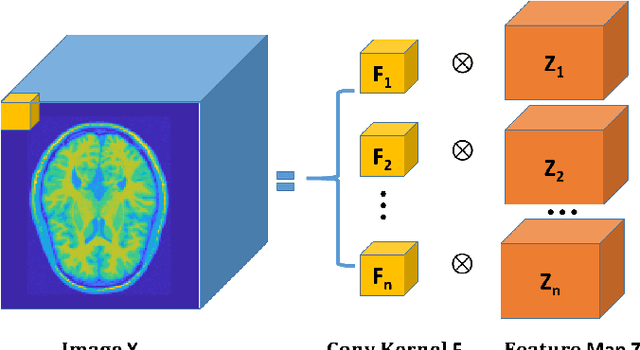
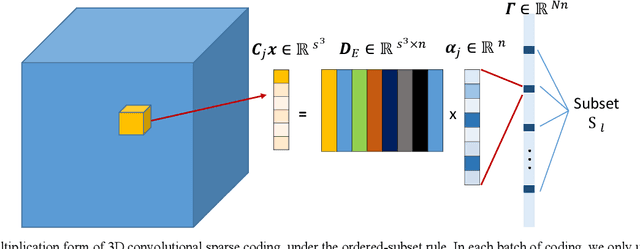
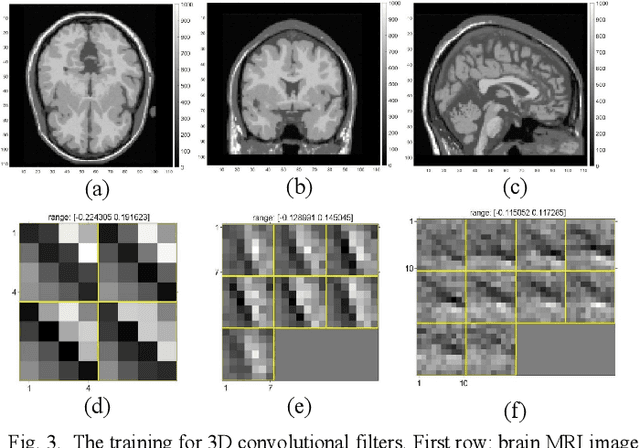
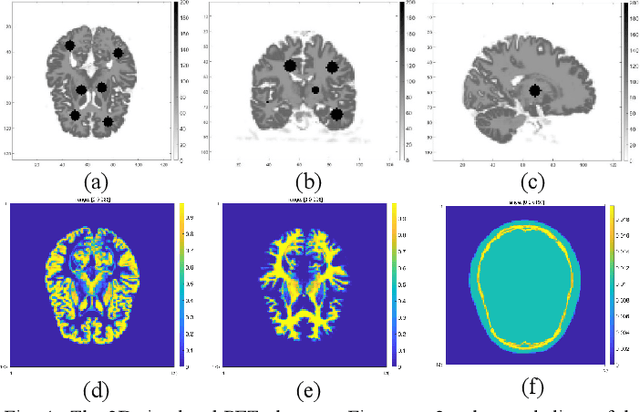
Abstract:Positron emission tomography (PET) is widely used for clinical diagnosis. As PET suffers from low resolution and high noise, numerous efforts try to incorporate anatomical priors into PET image reconstruction, especially with the development of hybrid PET/CT and PET/MRI systems. In this work, we proposed a novel 3D structural convolutional sparse coding (CSC) concept for penalized-likelihood PET image reconstruction, named 3D PET-CSC. The proposed 3D PET-CSC takes advantage of the convolutional operation and manages to incorporate anatomical priors without the need of registration or supervised training. As 3D PET-CSC codes the whole 3D PET image, instead of patches, it alleviates the staircase artifacts commonly presented in traditional patch-based sparse coding methods. Moreover, we developed the residual-image and order-subset mechanisms to further reduce the computational cost and accelerate the convergence for the proposed 3D PET-CSC method. Experiments based on computer simulations and clinical datasets demonstrate the superiority of 3D PET-CSC compared with other reference methods.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge