Zhaozhi Li
Massachusetts General Hospital, Boston, MA
Multi Scale Graph Neural Network for Alzheimer's Disease
Nov 16, 2024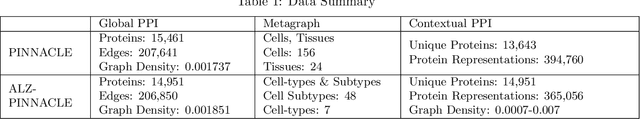
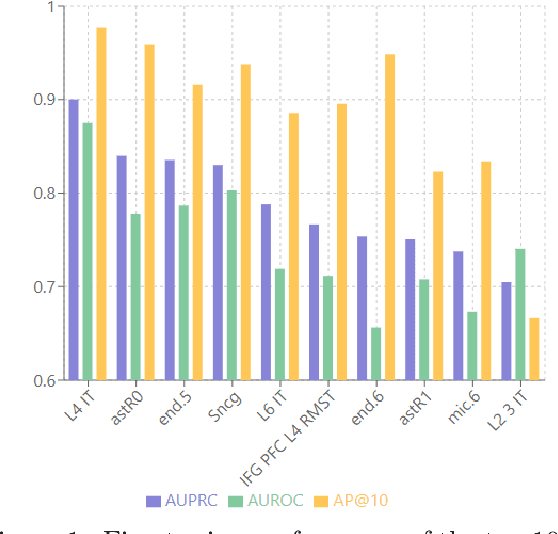
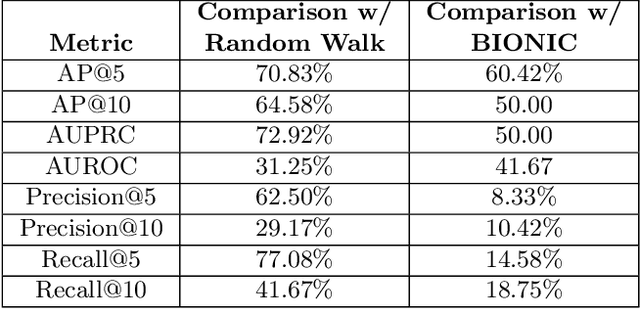
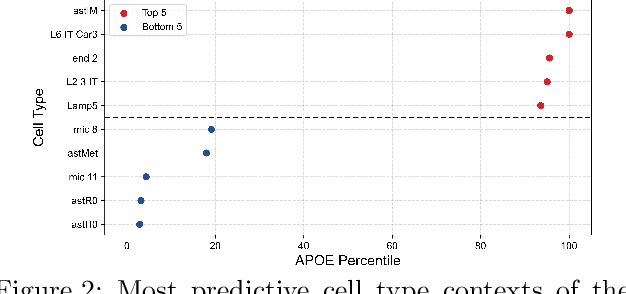
Abstract:Alzheimer's disease (AD) is a complex, progressive neurodegenerative disorder characterized by extracellular A\b{eta} plaques, neurofibrillary tau tangles, glial activation, and neuronal degeneration, involving multiple cell types and pathways. Current models often overlook the cellular context of these pathways. To address this, we developed a multiscale graph neural network (GNN) model, ALZ PINNACLE, using brain omics data from donors spanning the entire aging to AD spectrum. ALZ PINNACLE is based on the PINNACLE GNN framework, which learns context-aware protein, cell type, and tissue representations within a unified latent space. ALZ PINNACLE was trained on 14,951 proteins, 206,850 protein interactions, 7 cell types, and 48 cell subtypes or states. After pretraining, we investigated the learned embedding of APOE, the largest genetic risk factor for AD, across different cell types. Notably, APOE embeddings showed high similarity in microglial, neuronal, and CD8 cells, suggesting a similar role of APOE in these cell types. Fine tuning the model on AD risk genes revealed cell type contexts predictive of the role of APOE in AD. Our results suggest that ALZ PINNACLE may provide a valuable framework for uncovering novel insights into AD neurobiology.
Using Deep Learning to Identify Patients with Cognitive Impairment in Electronic Health Records
Nov 13, 2021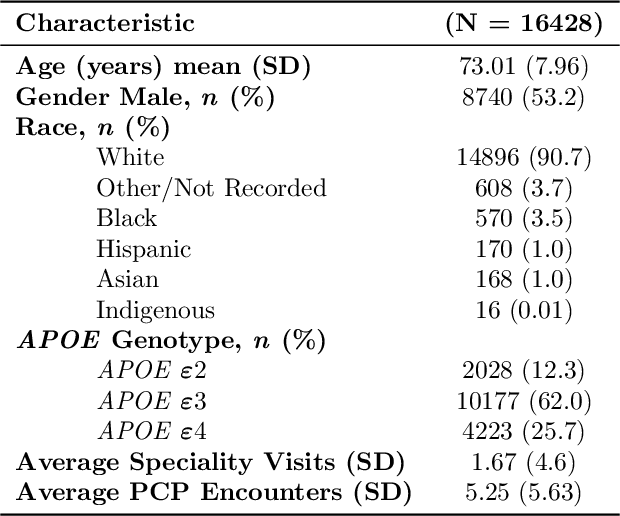
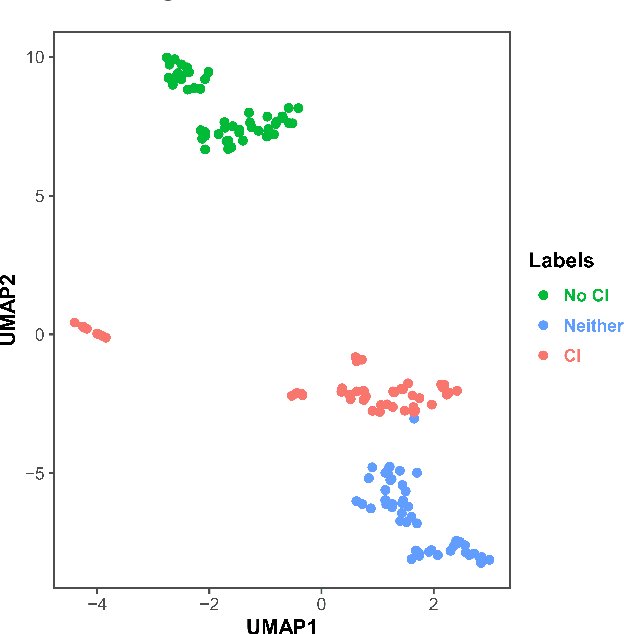

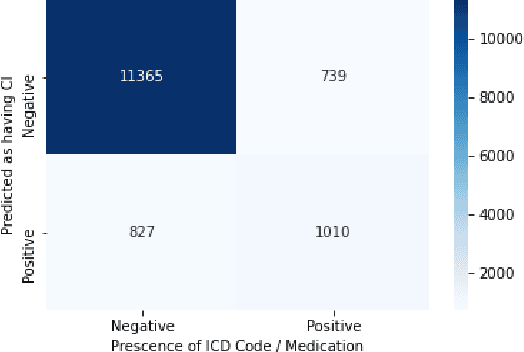
Abstract:Dementia is a neurodegenerative disorder that causes cognitive decline and affects more than 50 million people worldwide. Dementia is under-diagnosed by healthcare professionals - only one in four people who suffer from dementia are diagnosed. Even when a diagnosis is made, it may not be entered as a structured International Classification of Diseases (ICD) diagnosis code in a patient's charts. Information relevant to cognitive impairment (CI) is often found within electronic health records (EHR), but manual review of clinician notes by experts is both time consuming and often prone to errors. Automated mining of these notes presents an opportunity to label patients with cognitive impairment in EHR data. We developed natural language processing (NLP) tools to identify patients with cognitive impairment and demonstrate that linguistic context enhances performance for the cognitive impairment classification task. We fine-tuned our attention based deep learning model, which can learn from complex language structures, and substantially improved accuracy (0.93) relative to a baseline NLP model (0.84). Further, we show that deep learning NLP can successfully identify dementia patients without dementia-related ICD codes or medications.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge