Zeeshan Nisar
Maximising Histopathology Segmentation using Minimal Labels via Self-Supervision
Dec 19, 2024Abstract:Histopathology, the microscopic examination of tissue samples, is essential for disease diagnosis and prognosis. Accurate segmentation and identification of key regions in histopathology images are crucial for developing automated solutions. However, state-of-art deep learning segmentation methods like UNet require extensive labels, which is both costly and time-consuming, particularly when dealing with multiple stainings. To mitigate this, multi-stain segmentation methods such as MDS1 and UDAGAN have been developed, which reduce the need for labels by requiring only one (source) stain to be labelled. Nonetheless, obtaining source stain labels can still be challenging, and segmentation models fail when they are unavailable. This article shows that through self-supervised pre-training, including SimCLR, BYOL, and a novel approach, HR-CS-CO, the performance of these segmentation methods (UNet, MDS1, and UDAGAN) can be retained even with 95% fewer labels. Notably, with self-supervised pre-training and using only 5% labels, the performance drops are minimal: 5.9% for UNet, 4.5% for MDS1, and 6.2% for UDAGAN, compared to their respective fully supervised counterparts (without pre-training, using 100% labels). The code is available from https://github.com/zeeshannisar/improve_kidney_glomeruli_segmentation [to be made public upon acceptance].
Counterfactual Explanation and Instance-Generation using Cycle-Consistent Generative Adversarial Networks
Jan 21, 2023



Abstract:The image-based diagnosis is now a vital aspect of modern automation assisted diagnosis. To enable models to produce pixel-level diagnosis, pixel-level ground-truth labels are essentially required. However, since it is often not straight forward to obtain the labels in many application domains such as in medical image, classification-based approaches have become the de facto standard to perform the diagnosis. Though they can identify class-salient regions, they may not be useful for diagnosis where capturing all of the evidences is important requirement. Alternatively, a counterfactual explanation (CX) aims at providing explanations using a casual reasoning process of form "If X has not happend, Y would not heppend". Existing CX approaches, however, use classifier to explain features that can change its predictions. Thus, they can only explain class-salient features, rather than entire object of interest. This hence motivates us to propose a novel CX strategy that is not reliant on image classification. This work is inspired from the recent developments in generative adversarial networks (GANs) based image-to-image domain translation, and leverages to translate an abnormal image to counterpart normal image (i.e. counterfactual instance CI) to find discrepancy maps between the two. Since it is generally not possible to obtain abnormal and normal image pairs, we leverage Cycle-Consistency principle (a.k.a CycleGAN) to perform the translation in unsupervised way. We formulate CX in terms of a discrepancy map that, when added from the abnormal image, will make it indistinguishable from the CI. We evaluate our method on three datasets including a synthetic, tuberculosis and BraTS dataset. All these experiments confirm the supremacy of propose method in generating accurate CX and CI.
Towards Measuring Domain Shift in Histopathological Stain Translation in an Unsupervised Manner
May 09, 2022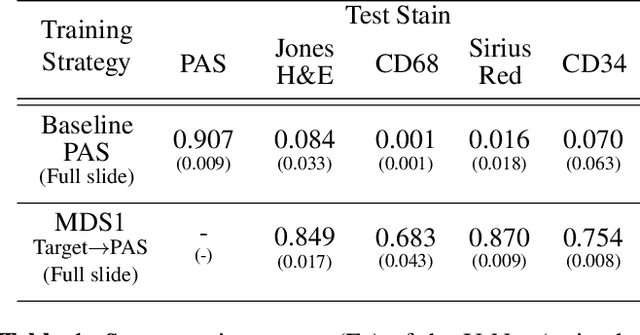
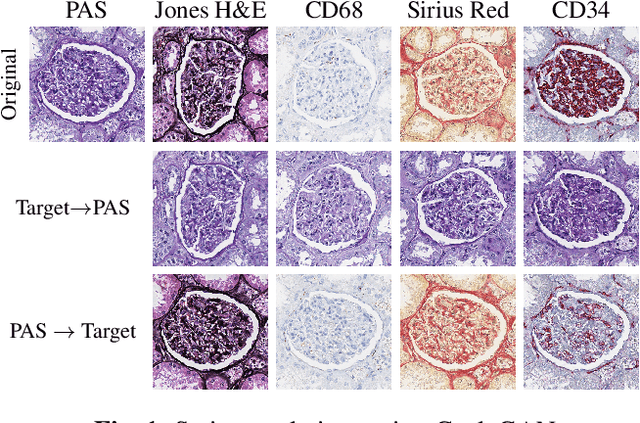
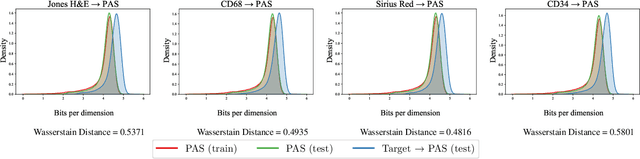
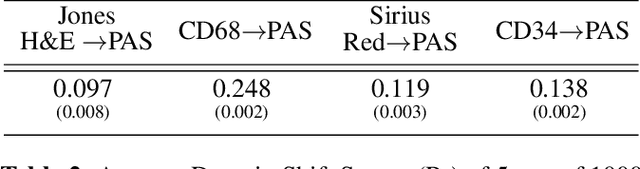
Abstract:Domain shift in digital histopathology can occur when different stains or scanners are used, during stain translation, etc. A deep neural network trained on source data may not generalise well to data that has undergone some domain shift. An important step towards being robust to domain shift is the ability to detect and measure it. This article demonstrates that the PixelCNN and domain shift metric can be used to detect and quantify domain shift in digital histopathology, and they demonstrate a strong correlation with generalisation performance. These findings pave the way for a mechanism to infer the average performance of a model (trained on source data) on unseen and unlabelled target data.
* 5 pages, 3 figures, 2 tables
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge