Yushan Feng
Prostate Segmentation from 3D MRI Using a Two-Stage Model and Variable-Input Based Uncertainty Measure
Mar 06, 2019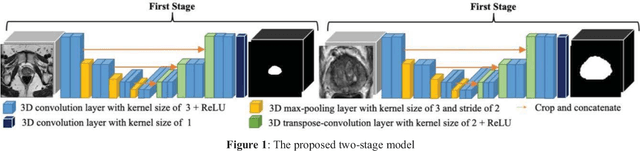
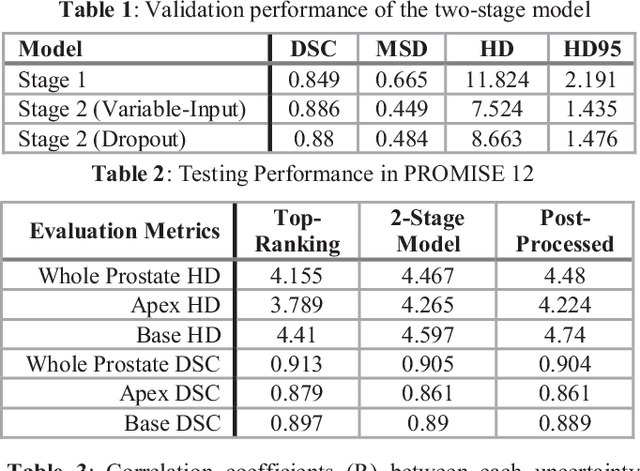
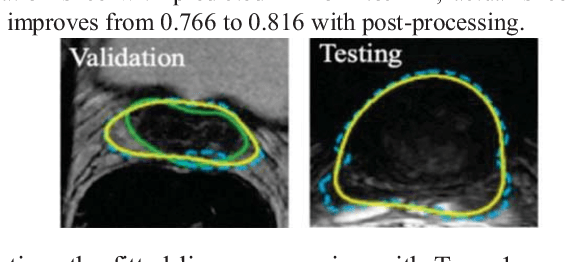
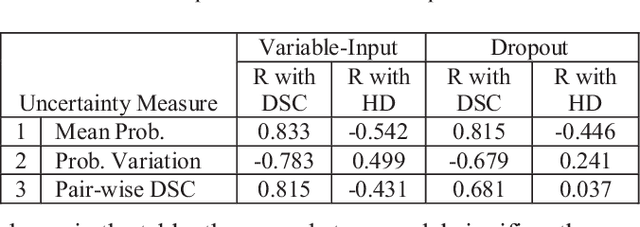
Abstract:This paper proposes a two-stage segmentation model, variable-input based uncertainty measures and an uncertainty-guided post-processing method for prostate segmentation on 3D magnetic resonance images (MRI). The two-stage model was based on 3D dilated U-Nets with the first stage to localize the prostate and the second stage to obtain an accurate segmentation from cropped images. For data augmentation, we proposed the variable-input method which crops the region of interest with additional random variations. Similar to other deep learning models, the proposed model also faced the challenge of suboptimal performance in certain testing cases due to varied training and testing image characteristics. Therefore, it is valuable to evaluate the confidence and performance of the network using uncertainty measures, which are often calculated from the probability maps or their standard deviations with multiple model outputs for the same testing case. However, few studies have quantitatively compared different methods of uncertainty calculation. Furthermore, unlike the commonly used Bayesian dropout during testing, we developed uncertainty measures based on the variable input images at the second stage and evaluated its performance by calculating the correlation with ground-truth-based performance metrics, such as Dice score. For performance estimation, we predicted Dice scores and Hausdorff distance with the most correlated uncertainty measure. For post-processing, we performed Gaussian filter on the underperformed slices to improve segmentation quality. Using PROMISE-12 data, we demonstrated the robustness of the two-stage model and showed high correlation of the proposed variable-input based uncertainty measures with GT-based performance. The uncertainty-guided post-processing method significantly improved label smoothness.
A Self-Adaptive Network For Multiple Sclerosis Lesion Segmentation From Multi-Contrast MRI With Various Imaging Protocols
Nov 19, 2018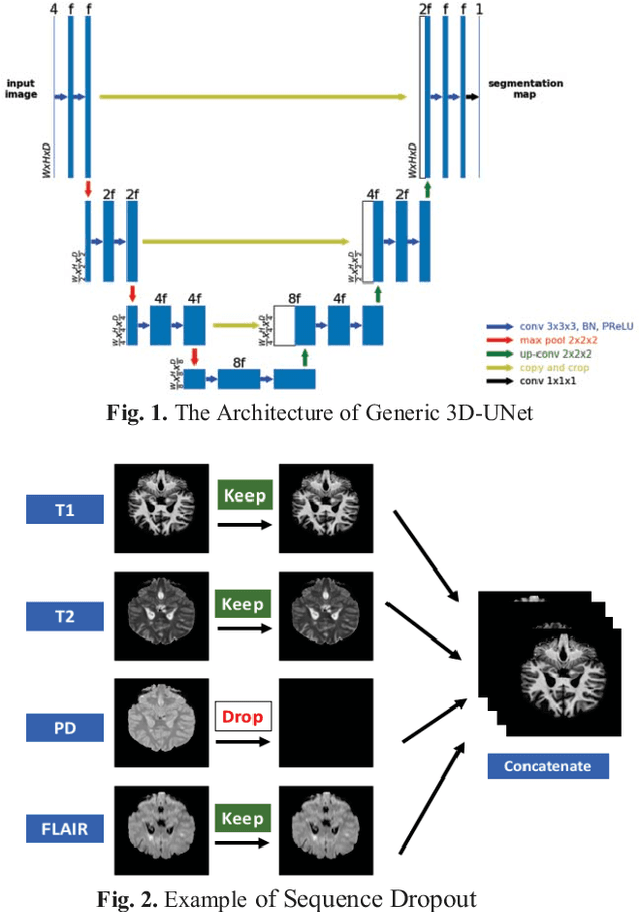
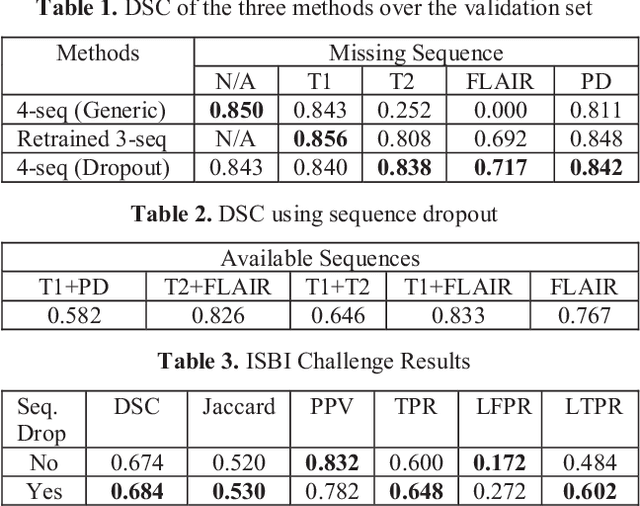
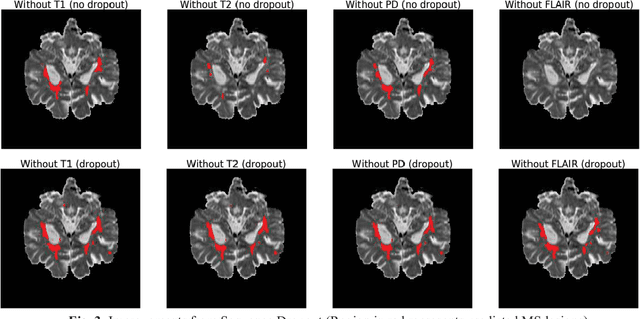
Abstract:Deep neural networks (DNN) have shown promises in the lesion segmentation of multiple sclerosis (MS) from multicontrast MRI including T1, T2, proton density (PD) and FLAIR sequences. However, one challenge in deploying such networks into clinical practice is the variability of imaging protocols, which often differ from the training dataset as certain MRI sequences may be unavailable or unusable. Therefore, trained networks need to adapt to practical situations when imaging protocols are different in deployment. In this paper, we propose a DNN-based MS lesion segmentation framework with a novel technique called sequence dropout which can adapt to various combinations of input MRI sequences during deployment and achieve the maximal possible performance from the given input. In addition, with this framework, we studied the quantitative impact of each MRI sequence on the MS lesion segmentation task without training separate networks. Experiments were performed using the IEEE ISBI 2015 Longitudinal MS Lesion Challenge dataset and our method is currently ranked 2nd with a Dice similarity coefficient of 0.684. Furthermore, we showed our network achieved the maximal possible performance when one sequence is unavailable during deployment by comparing with separate networks trained on the corresponding input MRI sequences. In particular, we discovered T1 and PD have minor impact on segmentation performance while FLAIR is the predominant sequence. Experiments with multiple missing sequences were also performed and showed the robustness of our network.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge