Vineet K Raghu
A Comparison of Self-Supervised Pretraining Approaches for Predicting Disease Risk from Chest Radiograph Images
Jun 15, 2023Abstract:Deep learning is the state-of-the-art for medical imaging tasks, but requires large, labeled datasets. For risk prediction, large datasets are rare since they require both imaging and follow-up (e.g., diagnosis codes). However, the release of publicly available imaging data with diagnostic labels presents an opportunity for self and semi-supervised approaches to improve label efficiency for risk prediction. Though several studies have compared self-supervised approaches in natural image classification, object detection, and medical image interpretation, there is limited data on which approaches learn robust representations for risk prediction. We present a comparison of semi- and self-supervised learning to predict mortality risk using chest x-ray images. We find that a semi-supervised autoencoder outperforms contrastive and transfer learning in internal and external validation.
A Pipeline for Integrated Theory and Data-Driven Modeling of Genomic and Clinical Data
May 05, 2020
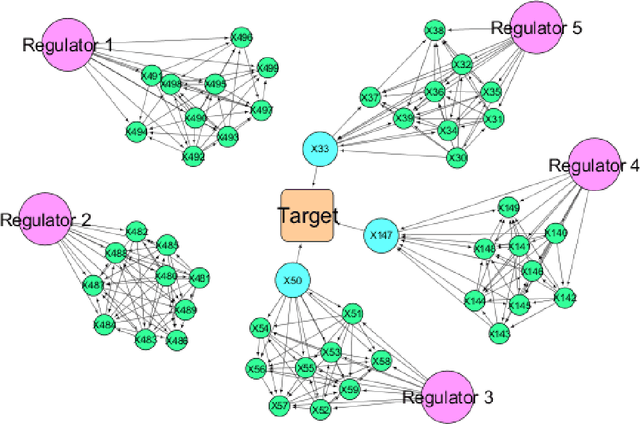
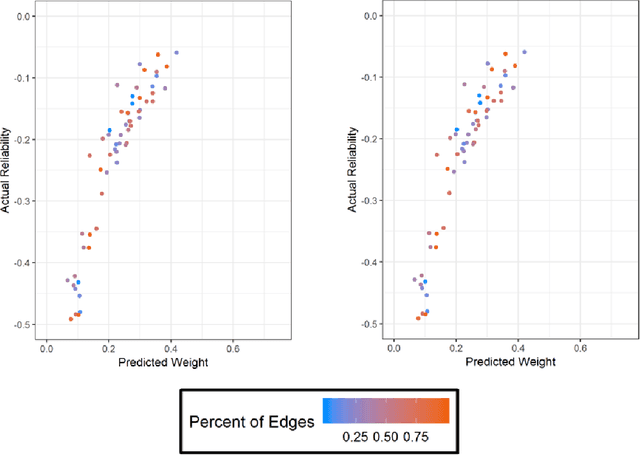
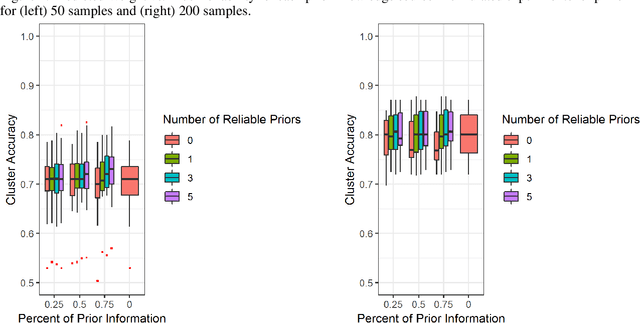
Abstract:High throughput genome sequencing technologies such as RNA-Seq and Microarray have the potential to transform clinical decision making and biomedical research by enabling high-throughput measurements of the genome at a granular level. However, to truly understand causes of disease and the effects of medical interventions, this data must be integrated with phenotypic, environmental, and behavioral data from individuals. Further, effective knowledge discovery methods that can infer relationships between these data types are required. In this work, we propose a pipeline for knowledge discovery from integrated genomic and clinical data. The pipeline begins with a novel variable selection method, and uses a probabilistic graphical model to understand the relationships between features in the data. We demonstrate how this pipeline can improve breast cancer outcome prediction models, and can provide a biologically interpretable view of sequencing data.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge