Vignesh Ravichandran
Deep Network for Capacitive ECG Denoising
Mar 29, 2019



Abstract:Continuous monitoring of cardiac health under free living condition is crucial to provide effective care for patients undergoing post operative recovery and individuals with high cardiac risk like the elderly. Capacitive Electrocardiogram (cECG) is one such technology which allows comfortable and long term monitoring through its ability to measure biopotential in conditions without having skin contact. cECG monitoring can be done using many household objects like chairs, beds and even car seats allowing for seamless monitoring of individuals. This method is unfortunately highly susceptible to motion artifacts which greatly limits its usage in clinical practice. The current use of cECG systems has been limited to performing rhythmic analysis. In this paper we propose a novel end-to-end deep learning architecture to perform the task of denoising capacitive ECG. The proposed network is trained using motion corrupted three channel cECG and a reference LEAD I ECG collected on individuals while driving a car. Further, we also propose a novel joint loss function to apply loss on both signal and frequency domain. We conduct extensive rhythmic analysis on the model predictions and the ground truth. We further evaluate the signal denoising using Mean Square Error(MSE) and Cross Correlation between model predictions and ground truth. We report MSE of 0.167 and Cross Correlation of 0.476. The reported results highlight the feasibility of performing morphological analysis using the filtered cECG. The proposed approach can allow for continuous and comprehensive monitoring of the individuals in free living conditions.
PPGnet: Deep Network for Device Independent Heart Rate Estimation from Photoplethysmogram
Mar 21, 2019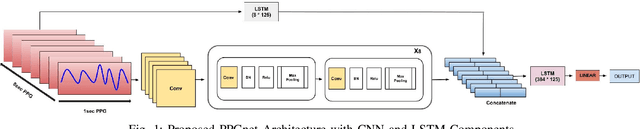



Abstract:Photoplethysmogram (PPG) is increasingly used to provide monitoring of the cardiovascular system under ambulatory conditions. Wearable devices like smartwatches use PPG to allow long term unobtrusive monitoring of heart rate in free living conditions. PPG based heart rate measurement is unfortunately highly susceptible to motion artifacts, particularly when measured from the wrist. Traditional machine learning and deep learning approaches rely on tri-axial accelerometer data along with PPG to perform heart rate estimation. The conventional learning based approaches have not addressed the need for device-specific modeling due to differences in hardware design among PPG devices. In this paper, we propose a novel end to end deep learning model to perform heart rate estimation using 8 second length input PPG signal. We evaluate the proposed model on the IEEE SPC 2015 dataset, achieving a mean absolute error of 3.36+-4.1BPM for HR estimation on 12 subjects without requiring patient specific training. We also studied the feasibility of applying transfer learning along with sparse retraining from a comprehensive in house PPG dataset for heart rate estimation across PPG devices with different hardware design.
RespNet: A deep learning model for extraction of respiration from photoplethysmogram
Feb 20, 2019



Abstract:Respiratory ailments afflict a wide range of people and manifests itself through conditions like asthma and sleep apnea. Continuous monitoring of chronic respiratory ailments is seldom used outside the intensive care ward due to the large size and cost of the monitoring system. While Electrocardiogram (ECG) based respiration extraction is a validated approach, its adoption is limited by access to a suitable continuous ECG monitor. Recently, due to the widespread adoption of wearable smartwatches with in-built Photoplethysmogram (PPG) sensor, it is being considered as a viable candidate for continuous and unobtrusive respiration monitoring. Research in this domain, however, has been predominantly focussed on estimating respiration rate from PPG. In this work, a novel end-to-end deep learning network called RespNet is proposed to perform the task of extracting the respiration signal from a given input PPG as opposed to extracting respiration rate. The proposed network was trained and tested on two different datasets utilizing different modalities of reference respiration signal recordings. Also, the similarity and performance of the proposed network against two conventional signal processing approaches for extracting respiration signal were studied. The proposed method was tested on two independent datasets with a Mean Squared Error of 0.262 and 0.145. The Cross-Correlation coefficient of the respective datasets were found to be 0.933 and 0.931. The reported errors and similarity was found to be better than conventional approaches. The proposed approach would aid clinicians to provide comprehensive evaluation of sleep-related respiratory conditions and chronic respiratory ailments while being comfortable and inexpensive for the patient.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge