Usama Sardar
Sequence-Based Nanobody-Antigen Binding Prediction
Jul 15, 2023



Abstract:Nanobodies (Nb) are monomeric heavy-chain fragments derived from heavy-chain only antibodies naturally found in Camelids and Sharks. Their considerably small size (~3-4 nm; 13 kDa) and favorable biophysical properties make them attractive targets for recombinant production. Furthermore, their unique ability to bind selectively to specific antigens, such as toxins, chemicals, bacteria, and viruses, makes them powerful tools in cell biology, structural biology, medical diagnostics, and future therapeutic agents in treating cancer and other serious illnesses. However, a critical challenge in nanobodies production is the unavailability of nanobodies for a majority of antigens. Although some computational methods have been proposed to screen potential nanobodies for given target antigens, their practical application is highly restricted due to their reliance on 3D structures. Moreover, predicting nanobodyantigen interactions (binding) is a time-consuming and labor-intensive task. This study aims to develop a machine-learning method to predict Nanobody-Antigen binding solely based on the sequence data. We curated a comprehensive dataset of Nanobody-Antigen binding and nonbinding data and devised an embedding method based on gapped k-mers to predict binding based only on sequences of nanobody and antigen. Our approach achieves up to 90% accuracy in binding prediction and is significantly more efficient compared to the widely-used computational docking technique.
Robust Brain Age Estimation via Regression Models and MRI-derived Features
Jun 08, 2023Abstract:The determination of biological brain age is a crucial biomarker in the assessment of neurological disorders and understanding of the morphological changes that occur during aging. Various machine learning models have been proposed for estimating brain age through Magnetic Resonance Imaging (MRI) of healthy controls. However, developing a robust brain age estimation (BAE) framework has been challenging due to the selection of appropriate MRI-derived features and the high cost of MRI acquisition. In this study, we present a novel BAE framework using the Open Big Healthy Brain (OpenBHB) dataset, which is a new multi-site and publicly available benchmark dataset that includes region-wise feature metrics derived from T1-weighted (T1-w) brain MRI scans of 3965 healthy controls aged between 6 to 86 years. Our approach integrates three different MRI-derived region-wise features and different regression models, resulting in a highly accurate brain age estimation with a Mean Absolute Error (MAE) of 3.25 years, demonstrating the framework's robustness. We also analyze our model's regression-based performance on gender-wise (male and female) healthy test groups. The proposed BAE framework provides a new approach for estimating brain age, which has important implications for the understanding of neurological disorders and age-related brain changes.
BioSequence2Vec: Efficient Embedding Generation For Biological Sequences
Apr 01, 2023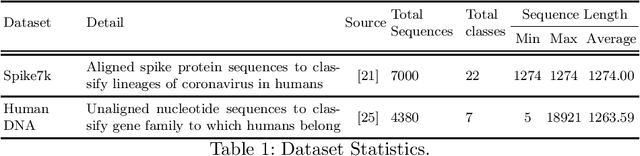


Abstract:Representation learning is an important step in the machine learning pipeline. Given the current biological sequencing data volume, learning an explicit representation is prohibitive due to the dimensionality of the resulting feature vectors. Kernel-based methods, e.g., SVM, are a proven efficient and useful alternative for several machine learning (ML) tasks such as sequence classification. Three challenges with kernel methods are (i) the computation time, (ii) the memory usage (storing an $n\times n$ matrix), and (iii) the usage of kernel matrices limited to kernel-based ML methods (difficult to generalize on non-kernel classifiers). While (i) can be solved using approximate methods, challenge (ii) remains for typical kernel methods. Similarly, although non-kernel-based ML methods can be applied to kernel matrices by extracting principal components (kernel PCA), it may result in information loss, while being computationally expensive. In this paper, we propose a general-purpose representation learning approach that embodies kernel methods' qualities while avoiding computation, memory, and generalizability challenges. This involves computing a low-dimensional embedding of each sequence, using random projections of its $k$-mer frequency vectors, significantly reducing the computation needed to compute the dot product and the memory needed to store the resulting representation. Our proposed fast and alignment-free embedding method can be used as input to any distance (e.g., $k$ nearest neighbors) and non-distance (e.g., decision tree) based ML method for classification and clustering tasks. Using different forms of biological sequences as input, we perform a variety of real-world classification tasks, such as SARS-CoV-2 lineage and gene family classification, outperforming several state-of-the-art embedding and kernel methods in predictive performance.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge