Tomer Meir
Heterogeneous Treatment Effect in Time-to-Event Outcomes: Harnessing Censored Data with Recursively Imputed Trees
Feb 03, 2025



Abstract:Tailoring treatments to individual needs is a central goal in fields such as medicine. A key step toward this goal is estimating Heterogeneous Treatment Effects (HTE) - the way treatments impact different subgroups. While crucial, HTE estimation is challenging with survival data, where time until an event (e.g., death) is key. Existing methods often assume complete observation, an assumption violated in survival data due to right-censoring, leading to bias and inefficiency. Cui et al. (2023) proposed a doubly-robust method for HTE estimation in survival data under no hidden confounders, combining a causal survival forest with an augmented inverse-censoring weighting estimator. However, we find it struggles under heavy censoring, which is common in rare-outcome problems such as Amyotrophic lateral sclerosis (ALS). Moreover, most current methods cannot handle instrumental variables, which are a crucial tool in the causal inference arsenal. We introduce Multiple Imputation for Survival Treatment Response (MISTR), a novel, general, and non-parametric method for estimating HTE in survival data. MISTR uses recursively imputed survival trees to handle censoring without directly modeling the censoring mechanism. Through extensive simulations and analysis of two real-world datasets-the AIDS Clinical Trials Group Protocol 175 and the Illinois unemployment dataset we show that MISTR outperforms prior methods under heavy censoring in the no-hidden-confounders setting, and extends to the instrumental variable setting. To our knowledge, MISTR is the first non-parametric approach for HTE estimation with unobserved confounders via instrumental variables.
Discrete-time Competing-Risks Regression with or without Penalization
Mar 02, 2023Abstract:Many studies employ the analysis of time-to-event data that incorporates competing risks and right censoring. Most methods and software packages are geared towards analyzing data that comes from a continuous failure time distribution. However, failure-time data may sometimes be discrete either because time is inherently discrete or due to imprecise measurement. This paper introduces a novel estimation procedure for discrete-time survival analysis with competing events. The proposed approach offers two key advantages over existing procedures: first, it accelerates the estimation process; second, it allows for straightforward integration and application of widely used regularized regression and screening methods. We illustrate the benefits of our proposed approach by conducting a comprehensive simulation study. Additionally, we showcase the utility of our procedure by estimating a survival model for the length of stay of patients hospitalized in the intensive care unit, considering three competing events: discharge to home, transfer to another medical facility, and in-hospital death.
PyDTS: A Python Package for Discrete Time Survival Analysis with Competing Risks
Apr 12, 2022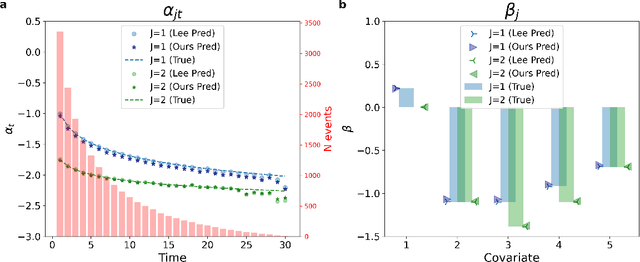
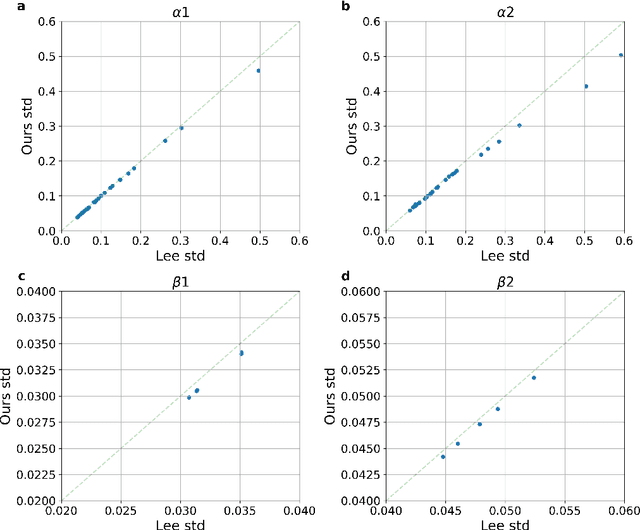
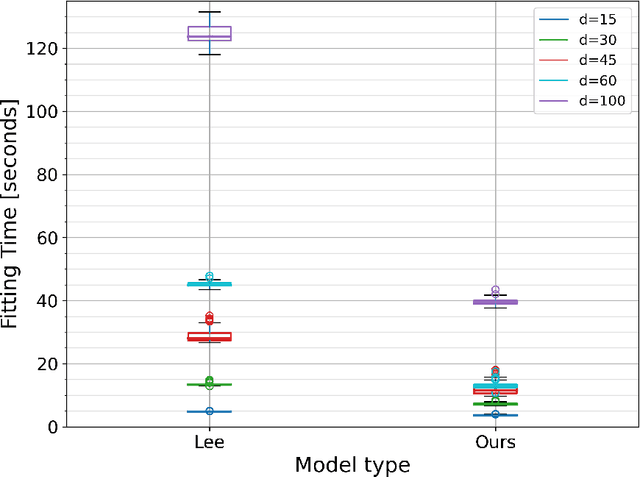
Abstract:Time-to-event analysis (survival analysis) is used when the outcome or the response of interest is the time until a pre-specified event occurs. Time-to-event data are sometimes discrete either because time itself is discrete or due to grouping of failure times into intervals or rounding off measurements. In addition, the failure of an individual could be one of several distinct failure types; known as competing risks (events) data. This work focuses on discrete-time regression with competing events. We emphasize the main difference between the continuous and discrete settings with competing events, develop a new estimation procedure, and present PyDTS, an open source Python package which implements our estimation procedure and other tools for discrete-time-survival analysis with competing risks.
3D Convolutional Sequence to Sequence Model for Vertebral Compression Fractures Identification in CT
Oct 08, 2020
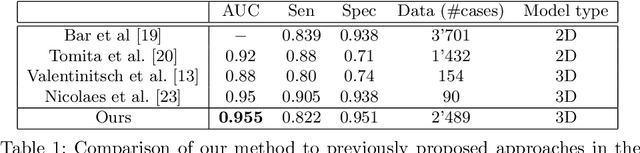


Abstract:An osteoporosis-related fracture occurs every three seconds worldwide, affecting one in three women and one in five men aged over 50. The early detection of at-risk patients facilitates effective and well-evidenced preventative interventions, reducing the incidence of major osteoporotic fractures. In this study, we present an automatic system for identification of vertebral compression fractures on Computed Tomography images, which are often an undiagnosed precursor to major osteoporosis-related fractures. The system integrates a compact 3D representation of the spine, utilizing a Convolutional Neural Network (CNN) for spinal cord detection and a novel end-to-end sequence to sequence 3D architecture. We evaluate several model variants that exploit different representation and classification approaches and present a framework combining an ensemble of models that achieves state of the art results, validated on a large data set, with a patient-level fracture identification of 0.955 Area Under the Curve (AUC). The system proposed has the potential to support osteoporosis clinical management, improve treatment pathways, and to change the course of one of the most burdensome diseases of our generation.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge