Tathagato Rai Dastidar
Rapid Training Data Creation by Synthesizing Medical Images for Classification and Localization
Aug 09, 2023Abstract:While the use of artificial intelligence (AI) for medical image analysis is gaining wide acceptance, the expertise, time and cost required to generate annotated data in the medical field are significantly high, due to limited availability of both data and expert annotation. Strongly supervised object localization models require data that is exhaustively annotated, meaning all objects of interest in an image are identified. This is difficult to achieve and verify for medical images. We present a method for the transformation of real data to train any Deep Neural Network to solve the above problems. We show the efficacy of this approach on both a weakly supervised localization model and a strongly supervised localization model. For the weakly supervised model, we show that the localization accuracy increases significantly using the generated data. For the strongly supervised model, this approach overcomes the need for exhaustive annotation on real images. In the latter model, we show that the accuracy, when trained with generated images, closely parallels the accuracy when trained with exhaustively annotated real images. The results are demonstrated on images of human urine samples obtained using microscopy.
Target-Independent Domain Adaptation for WBC Classification using Generative Latent Search
May 11, 2020



Abstract:Automating the classification of camera-obtained microscopic images of White Blood Cells (WBCs) and related cell subtypes has assumed importance since it aids the laborious manual process of review and diagnosis. Several State-Of-The-Art (SOTA) methods developed using Deep Convolutional Neural Networks suffer from the problem of domain shift - severe performance degradation when they are tested on data (target) obtained in a setting different from that of the training (source). The change in the target data might be caused by factors such as differences in camera/microscope types, lenses, lighting-conditions etc. This problem can potentially be solved using Unsupervised Domain Adaptation (UDA) techniques albeit standard algorithms presuppose the existence of a sufficient amount of unlabelled target data which is not always the case with medical images. In this paper, we propose a method for UDA that is devoid of the need for target data. Given a test image from the target data, we obtain its 'closest-clone' from the source data that is used as a proxy in the classifier. We prove the existence of such a clone given that infinite number of data points can be sampled from the source distribution. We propose a method in which a latent-variable generative model based on variational inference is used to simultaneously sample and find the 'closest-clone' from the source distribution through an optimization procedure in the latent space. We demonstrate the efficacy of the proposed method over several SOTA UDA methods for WBC classification on datasets captured using different imaging modalities under multiple settings.
Learning a Deep Convolution Network with Turing Test Adversaries for Microscopy Image Super Resolution
Jan 18, 2019
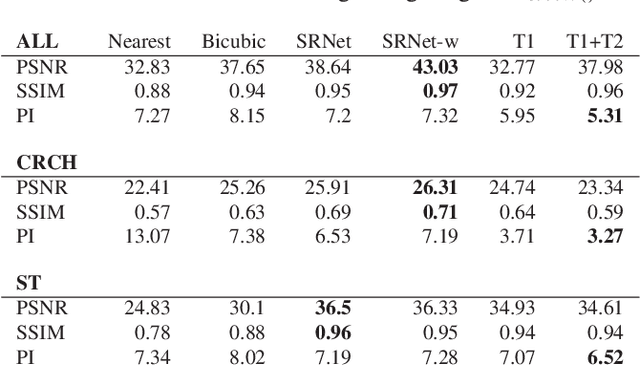
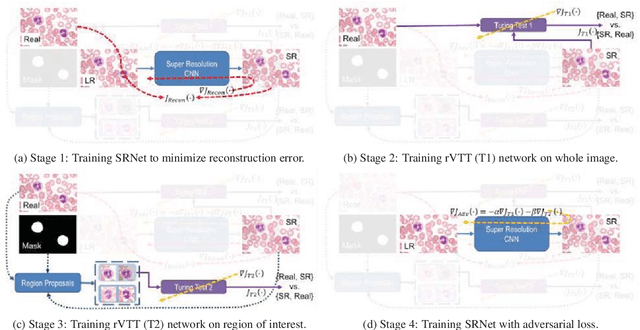

Abstract:Adversarially trained deep neural networks have significantly improved performance of single image super resolution, by hallucinating photorealistic local textures, thereby greatly reducing the perception difference between a real high resolution image and its super resolved (SR) counterpart. However, application to medical imaging requires preservation of diagnostically relevant features while refraining from introducing any diagnostically confusing artifacts. We propose using a deep convolutional super resolution network (SRNet) trained for (i) minimising reconstruction loss between the real and SR images, and (ii) maximally confusing learned relativistic visual Turing test (rVTT) networks to discriminate between (a) pair of real and SR images (T1) and (b) pair of patches in real and SR selected from region of interest (T2). The adversarial loss of T1 and T2 while backpropagated through SRNet helps it learn to reconstruct pathorealism in the regions of interest such as white blood cells (WBC) in peripheral blood smears or epithelial cells in histopathology of cancerous biopsy tissues, which are experimentally demonstrated here. Experiments performed for measuring signal distortion loss using peak signal to noise ratio (pSNR) and structural similarity (SSIM) with variation of SR scale factors, impact of rVTT adversarial losses, and impact on reporting using SR on a commercially available artificial intelligence (AI) digital pathology system substantiate our claims.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge