Steffen Schuler
Non-invasive Localization of the Ventricular Excitation Origin Without Patient-specific Geometries Using Deep Learning
Sep 16, 2022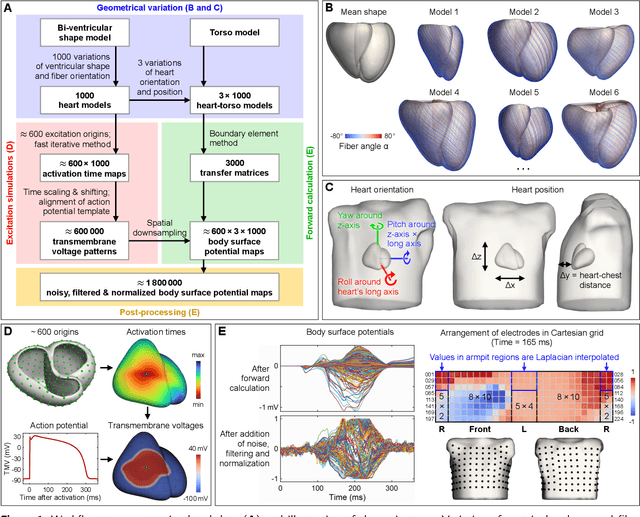

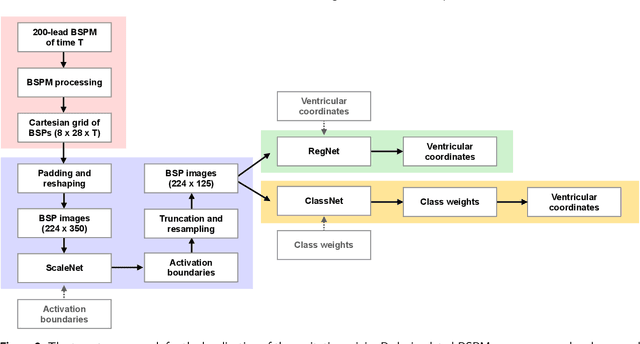
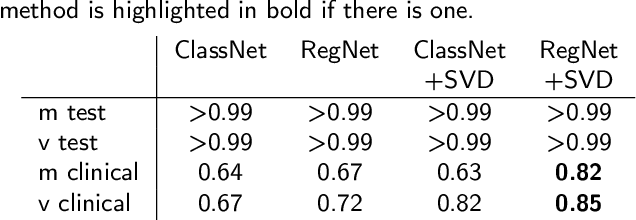
Abstract:Ventricular tachycardia (VT) can be one cause of sudden cardiac death affecting 4.25 million persons per year worldwide. A curative treatment is catheter ablation in order to inactivate the abnormally triggering regions. To facilitate and expedite the localization during the ablation procedure, we present two novel localization techniques based on convolutional neural networks (CNNs). In contrast to existing methods, e.g. using ECG imaging, our approaches were designed to be independent of the patient-specific geometries and directly applicable to surface ECG signals, while also delivering a binary transmural position. One method outputs ranked alternative solutions. Results can be visualized either on a generic or patient geometry. The CNNs were trained on a data set containing only simulated data and evaluated both on simulated and clinical test data. On simulated data, the median test error was below 3mm. The median localization error on the clinical data was as low as 32mm. The transmural position was correctly detected in up to 82% of all clinical cases. Using the ranked alternative solutions, the top-3 median error dropped to 20mm on clinical data. These results demonstrate a proof of principle to utilize CNNs to localize the activation source without the intrinsic need of patient-specific geometrical information. Furthermore, delivering multiple solutions can help the physician to find the real activation source amongst more than one possible locations. With further optimization, these methods have a high potential to speed up clinical interventions. Consequently they could decrease procedural risk and improve VT patients' outcomes.
Reducing Line-of-block Artifacts in Cardiac Activation Maps Estimated Using ECG Imaging: A Comparison of Source Models and Estimation Methods
Aug 18, 2021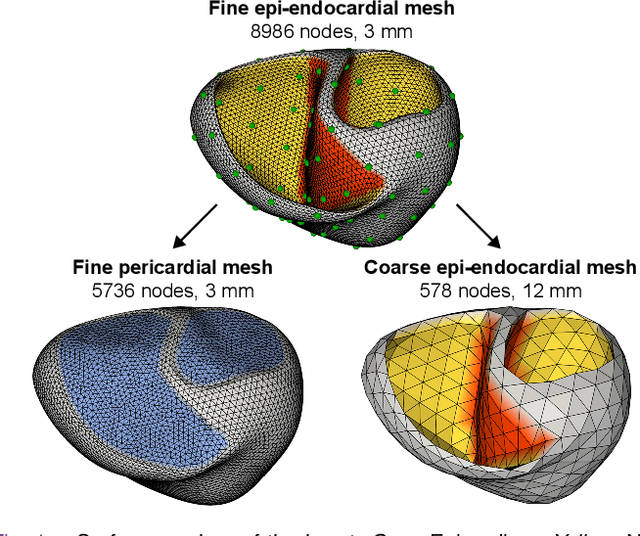
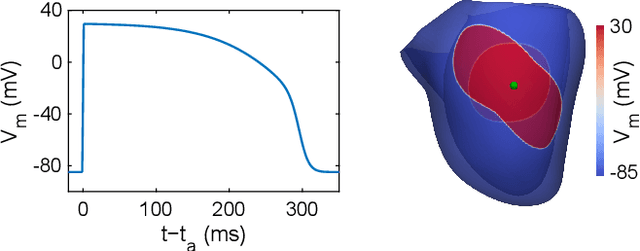
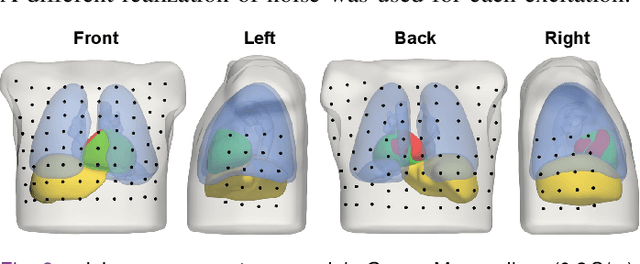
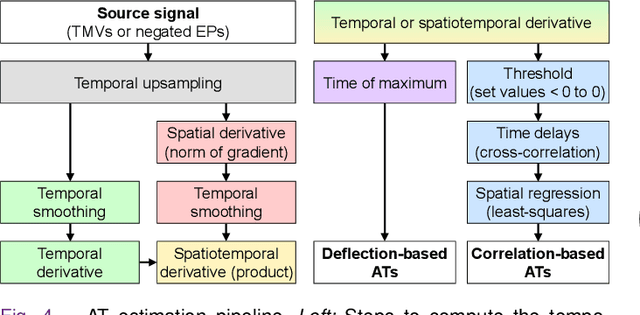
Abstract:Objective: To investigate cardiac activation maps estimated using electrocardiographic imaging and to find methods reducing line-of-block (LoB) artifacts, while preserving real LoBs. Methods: Body surface potentials were computed for 137 simulated ventricular excitations. Subsequently, the inverse problem was solved to obtain extracellular potentials (EP) and transmembrane voltages (TMV). From these, activation times (AT) were estimated using four methods and compared to the ground truth. This process was evaluated with two cardiac mesh resolutions. Factors contributing to LoB artifacts were identified by analyzing the impact of spatial and temporal smoothing on the morphology of source signals. Results: AT estimation using a spatiotemporal derivative performed better than using a temporal derivative. Compared to deflection-based AT estimation, correlation-based methods were less prone to LoB artifacts but performed worse in identifying real LoBs. Temporal smoothing could eliminate artifacts for TMVs but not for EPs, which could be linked to their temporal morphology. TMVs led to more accurate ATs on the septum than EPs. Mesh resolution had a negligible effect on inverse reconstructions, but small distances were important for cross-correlation-based estimation of AT delays. Conclusion: LoB artifacts are mainly caused by the inherent spatial smoothing effect of the inverse reconstruction. Among the configurations evaluated, only deflection-based AT estimation in combination with TMVs and strong temporal smoothing can prevent LoB artifacts, while preserving real LoBs. Significance: Regions of slow conduction are of considerable clinical interest and LoB artifacts observed in non-invasive ATs can lead to misinterpretations. We addressed this problem by identifying factors causing such artifacts and methods to reduce them.
A bi-atrial statistical shape model for large-scale in silico studies of human atria: model development and application to ECG simulations
Feb 22, 2021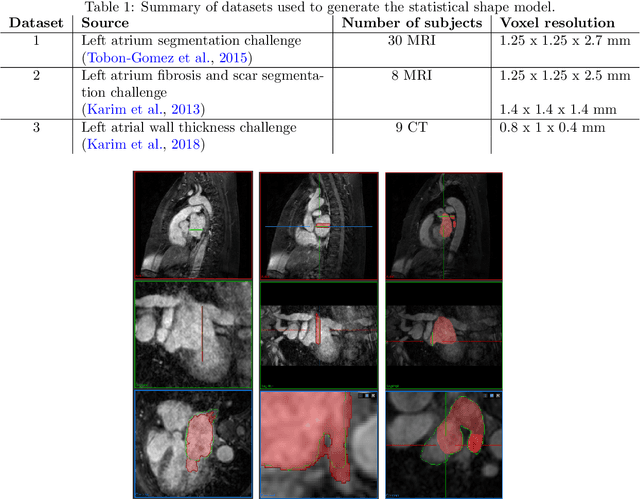
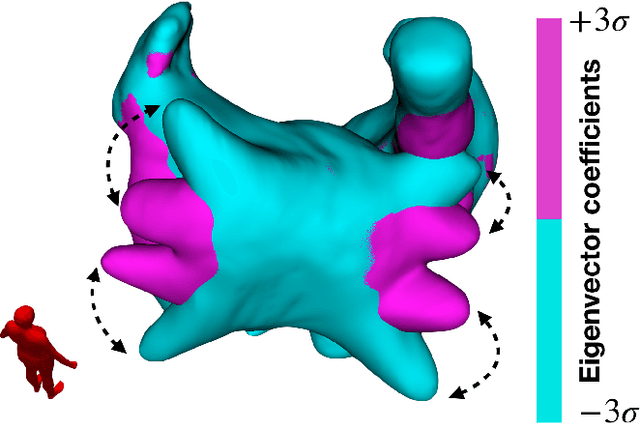
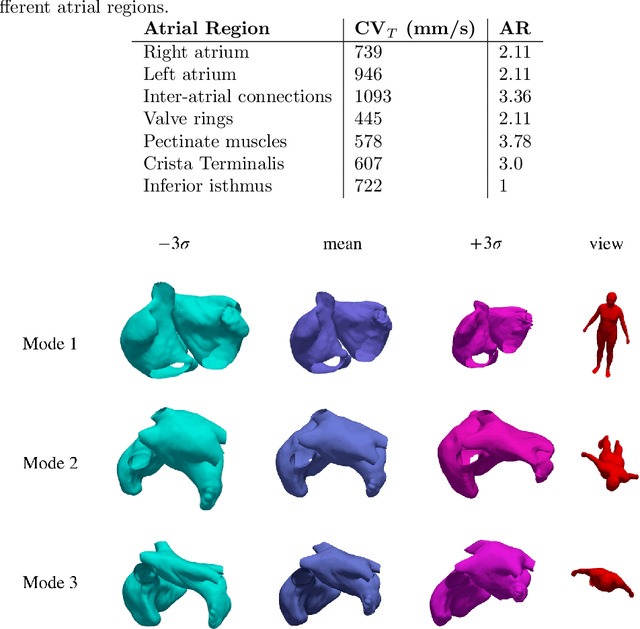
Abstract:Large-scale electrophysiological simulations to obtain electrocardiograms (ECG) carry the potential to produce extensive datasets for training of machine learning classifiers to, e.g., discriminate between different cardiac pathologies. The adoption of simulations for these purposes is limited due to a lack of ready-to-use models covering atrial anatomical variability. We built a bi-atrial statistical shape model (SSM) of the endocardial wall based on 47 segmented human CT and MRI datasets using Gaussian process morphable models. Generalization, specificity, and compactness metrics were evaluated. The SSM was applied to simulate atrial ECGs in 100 random volumetric instances. The first eigenmode of our SSM reflects a change of the total volume of both atria, the second the asymmetry between left vs. right atrial volume, the third a change in the prominence of the atrial appendages. The SSM is capable of generalizing well to unseen geometries and 95% of the total shape variance is covered by its first 23 eigenvectors. The P waves in the 12-lead ECG of 100 random instances showed a duration of 104ms in accordance with large cohort studies. The novel bi-atrial SSM itself as well as 100 exemplary instances with rule-based augmentation of atrial wall thickness, fiber orientation, inter-atrial bridges and tags for anatomical structures have been made publicly available. The novel, openly available bi-atrial SSM can in future be employed to generate large sets of realistic atrial geometries as a basis for in silico big data approaches.
Cobiveco: Consistent biventricular coordinates for precise and intuitive description of position in the heart -- with MATLAB implementation
Feb 04, 2021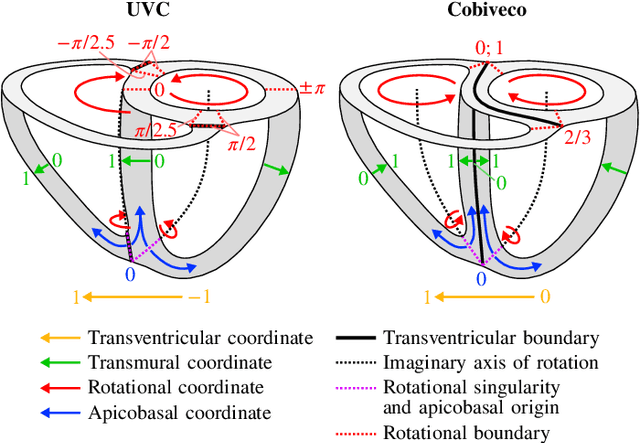
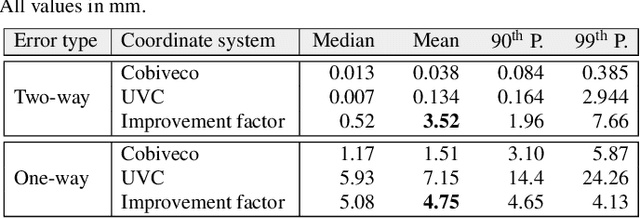
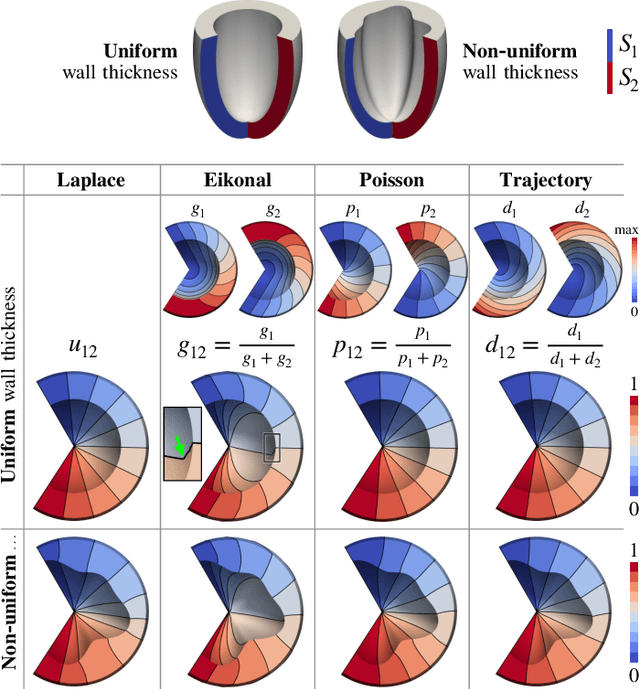
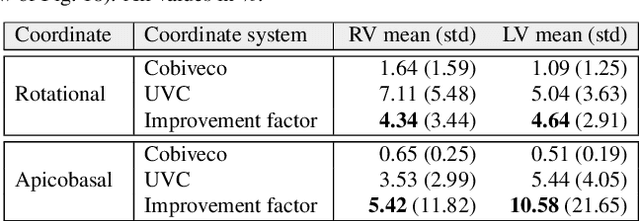
Abstract:Ventricular coordinates are widely used as a versatile tool for various applications that benefit from a description of local position within the heart. However, the practical usefulness of ventricular coordinates is determined by their ability to meet application-specific requirements. For regression-based estimation of biventricular position, for example, a consistent definition of coordinate directions in both ventricles is important. For the transfer of data between different hearts as another use case, the coordinate values are required to be consistent across different geometries. Existing ventricular coordinate systems do not meet these requirements. We first compare different approaches to compute coordinates and then present Cobiveco, a consistent and intuitive biventricular coordinate system to overcome these drawbacks. A novel one-way mapping error is introduced to assess the consistency of the coordinates. Evaluation of mapping and linearity errors on 36 patient geometries showed a more than 4-fold improvement compared to a state-of-the-art method. Finally, we show two application examples underlining the relevance for cardiac data processing. Cobiveco MATLAB code is available under a permissive open-source license.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge