Matthias Schaufelberger
Impact of Data Synthesis Strategies for the Classification of Craniosynostosis
Oct 16, 2023



Abstract:Introduction: Photogrammetric surface scans provide a radiation-free option to assess and classify craniosynostosis. Due to the low prevalence of craniosynostosis and high patient restrictions, clinical data is rare. Synthetic data could support or even replace clinical data for the classification of craniosynostosis, but this has never been studied systematically. Methods: We test the combinations of three different synthetic data sources: a statistical shape model (SSM), a generative adversarial network (GAN), and image-based principal component analysis for a convolutional neural network (CNN)-based classification of craniosynostosis. The CNN is trained only on synthetic data, but validated and tested on clinical data. Results: The combination of a SSM and a GAN achieved an accuracy of more than 0.96 and a F1-score of more than 0.95 on the unseen test set. The difference to training on clinical data was smaller than 0.01. Including a second image modality improved classification performance for all data sources. Conclusion: Without a single clinical training sample, a CNN was able to classify head deformities as accurate as if it was trained on clinical data. Using multiple data sources was key for a good classification based on synthetic data alone. Synthetic data might play an important future role in the assessment of craniosynostosis.
A statistical shape model for radiation-free assessment and classification of craniosynostosis
Jan 10, 2022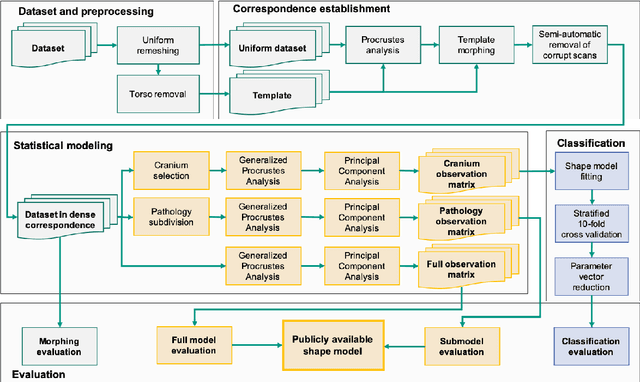

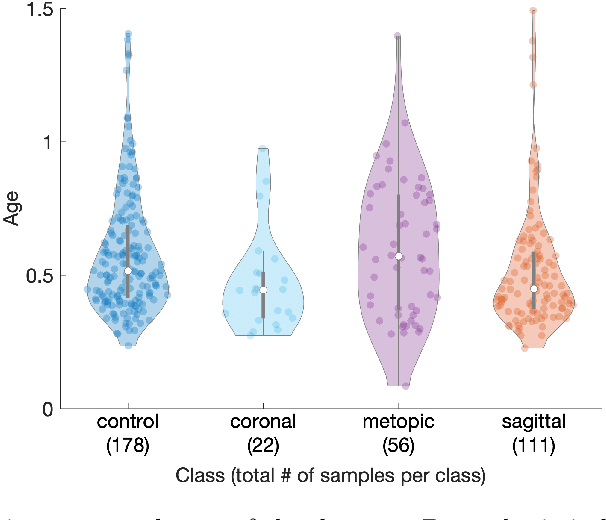

Abstract:The assessment of craniofacial deformities requires patient data which is sparsely available. Statistical shape models provide realistic and synthetic data enabling comparisons of existing methods on a common dataset. We build the first publicly available statistical 3D head model of craniosynostosis patients and the first model focusing on infants younger than 1.5 years. For correspondence establishment, we test and evaluate four template morphing approaches. We further present an original, shape-model-based classification approach for craniosynostosis on photogrammetric surface scans. To the best of our knowledge, our study uses the largest dataset of craniosynostosis patients in a classification study for craniosynostosis and statistical shape modeling to date. We demonstrate that our shape model performs similar to other statistical shape models of the human head. Craniosynostosis-specific pathologies are represented in the first eigenmodes of the model. Regarding the automatic classification of craniosynostis, our classification approach yields an accuracy of 97.3%, comparable to other state-of-the-art methods using both computed tomography scans and stereophotogrammetry. Our publicly available, craniosynostosis-specific statistical shape model enables the assessment of craniosynostosis on realistic and synthetic data. We further present a state-of-the-art shape-model-based classification approach for a radiation-free diagnosis of craniosynostosis.
Reducing Line-of-block Artifacts in Cardiac Activation Maps Estimated Using ECG Imaging: A Comparison of Source Models and Estimation Methods
Aug 18, 2021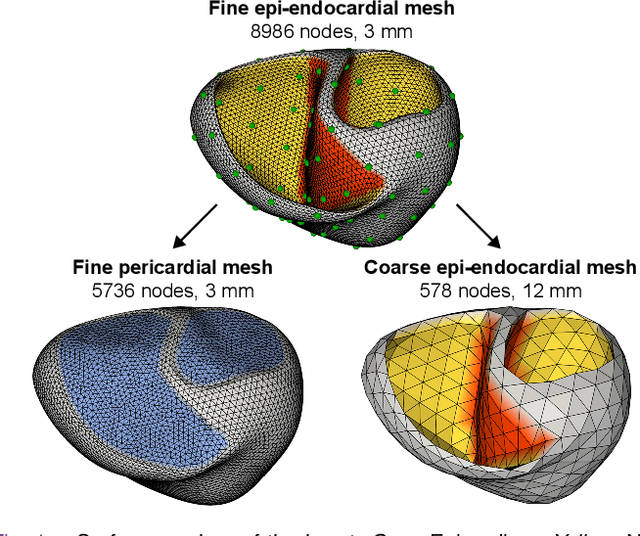
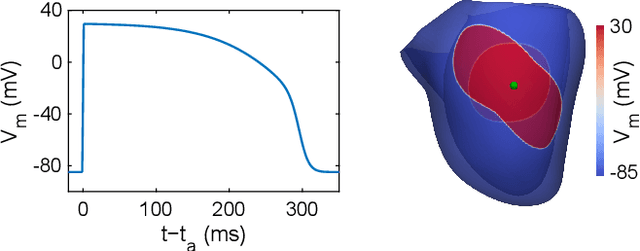
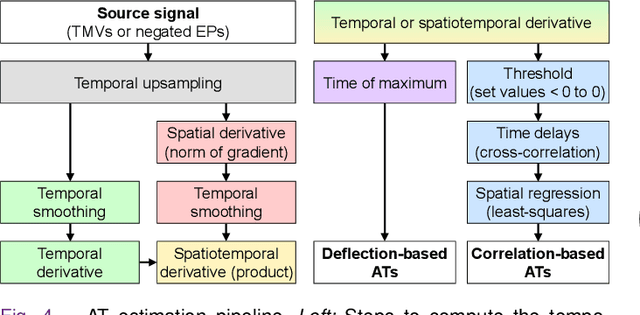
Abstract:Objective: To investigate cardiac activation maps estimated using electrocardiographic imaging and to find methods reducing line-of-block (LoB) artifacts, while preserving real LoBs. Methods: Body surface potentials were computed for 137 simulated ventricular excitations. Subsequently, the inverse problem was solved to obtain extracellular potentials (EP) and transmembrane voltages (TMV). From these, activation times (AT) were estimated using four methods and compared to the ground truth. This process was evaluated with two cardiac mesh resolutions. Factors contributing to LoB artifacts were identified by analyzing the impact of spatial and temporal smoothing on the morphology of source signals. Results: AT estimation using a spatiotemporal derivative performed better than using a temporal derivative. Compared to deflection-based AT estimation, correlation-based methods were less prone to LoB artifacts but performed worse in identifying real LoBs. Temporal smoothing could eliminate artifacts for TMVs but not for EPs, which could be linked to their temporal morphology. TMVs led to more accurate ATs on the septum than EPs. Mesh resolution had a negligible effect on inverse reconstructions, but small distances were important for cross-correlation-based estimation of AT delays. Conclusion: LoB artifacts are mainly caused by the inherent spatial smoothing effect of the inverse reconstruction. Among the configurations evaluated, only deflection-based AT estimation in combination with TMVs and strong temporal smoothing can prevent LoB artifacts, while preserving real LoBs. Significance: Regions of slow conduction are of considerable clinical interest and LoB artifacts observed in non-invasive ATs can lead to misinterpretations. We addressed this problem by identifying factors causing such artifacts and methods to reduce them.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge