Stefanos Apostolopoulos
GLAMpoints: Greedily Learned Accurate Match points
Aug 19, 2019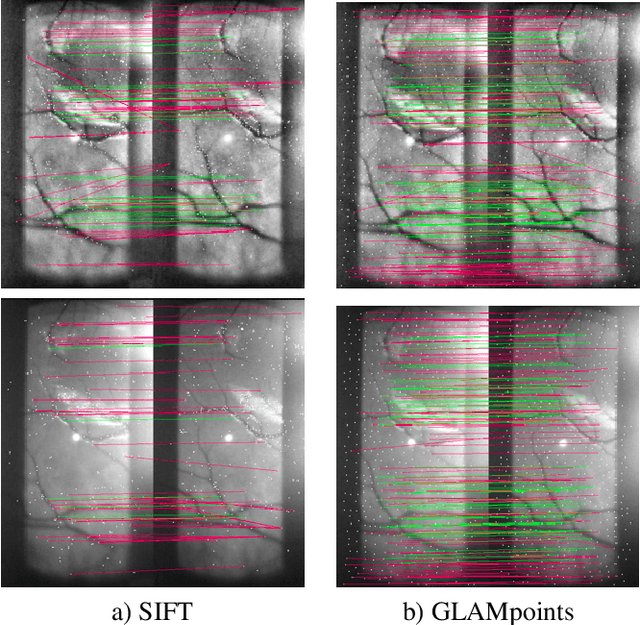
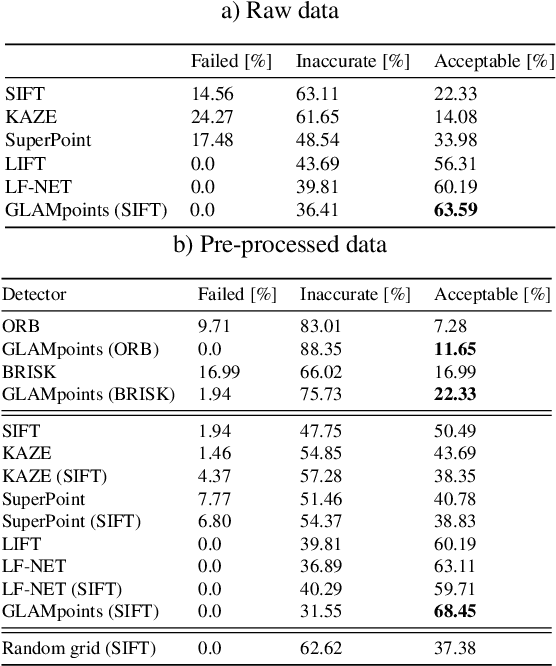
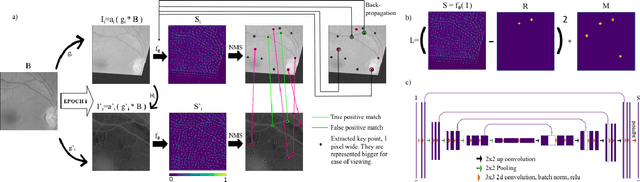
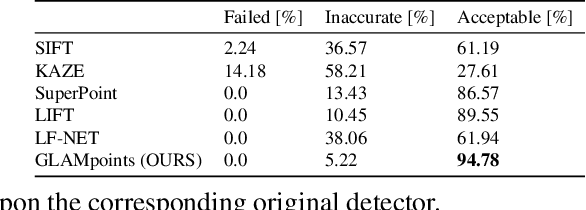
Abstract:We introduce a novel CNN-based feature point detector - GLAMpoints - learned in a semi-supervised manner. Our detector extracts repeatable, stable interest points with a dense coverage, specifically designed to maximize the correct matching in a specific domain, which is in contrast to conventional techniques that optimize indirect metrics. In this paper, we apply our method on challenging retinal slitlamp images, for which classical detectors yield unsatisfactory results due to low image quality and insufficient amount of low-level features. We show that GLAMpoints significantly outperforms classical detectors as well as state-of-the-art CNN-based methods in matching and registration quality for retinal images.
Pathological OCT Retinal Layer Segmentation using Branch Residual U-shape Networks
Jul 16, 2017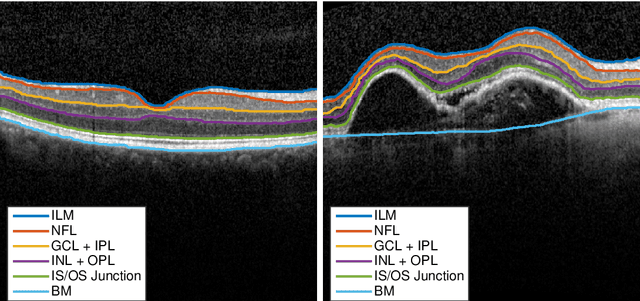
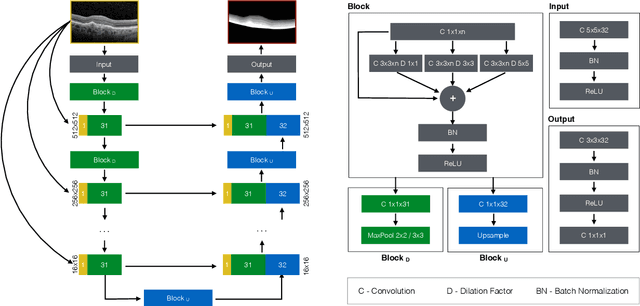
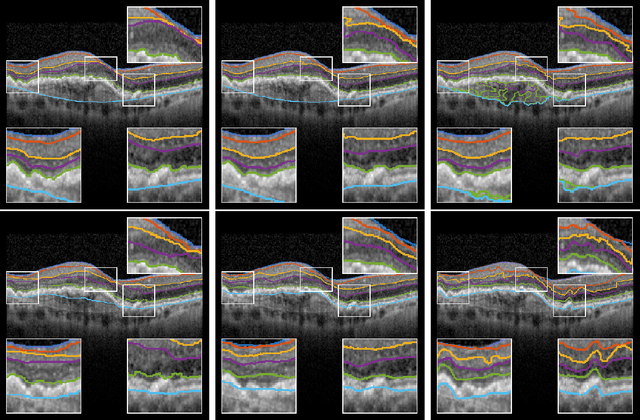
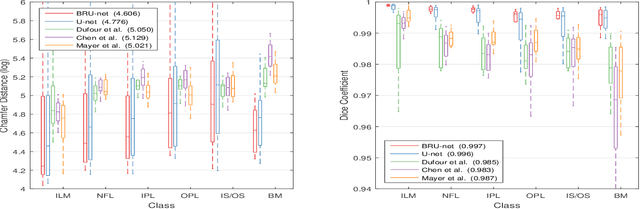
Abstract:The automatic segmentation of retinal layer structures enables clinically-relevant quantification and monitoring of eye disorders over time in OCT imaging. Eyes with late-stage diseases are particularly challenging to segment, as their shape is highly warped due to pathological biomarkers. In this context, we propose a novel fully Convolutional Neural Network (CNN) architecture which combines dilated residual blocks in an asymmetric U-shape configuration, and can segment multiple layers of highly pathological eyes in one shot. We validate our approach on a dataset of late-stage AMD patients and demonstrate lower computational costs and higher performance compared to other state-of-the-art methods.
RetiNet: Automatic AMD identification in OCT volumetric data
Oct 12, 2016



Abstract:Optical Coherence Tomography (OCT) provides a unique ability to image the eye retina in 3D at micrometer resolution and gives ophthalmologist the ability to visualize retinal diseases such as Age-Related Macular Degeneration (AMD). While visual inspection of OCT volumes remains the main method for AMD identification, doing so is time consuming as each cross-section within the volume must be inspected individually by the clinician. In much the same way, acquiring ground truth information for each cross-section is expensive and time consuming. This fact heavily limits the ability to acquire large amounts of ground truth, which subsequently impacts the performance of learning-based methods geared at automatic pathology identification. To avoid this burden, we propose a novel strategy for automatic analysis of OCT volumes where only volume labels are needed. That is, we train a classifier in a semi-supervised manner to conduct this task. Our approach uses a novel Convolutional Neural Network (CNN) architecture, that only needs volume-level labels to be trained to automatically asses whether an OCT volume is healthy or contains AMD. Our architecture involves first learning a cross-section pathology classifier using pseudo-labels that could be corrupted and then leverage these towards a more accurate volume-level classification. We then show that our approach provides excellent performances on a publicly available dataset and outperforms a number of existing automatic techniques.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge