Silvio H. Litovsky
SCPAT-GAN: Structural Constrained and Pathology Aware Convolutional Transformer-GAN for Virtual Histology Staining of Human Coronary OCT images
Jul 22, 2023Abstract:There is a significant need for the generation of virtual histological information from coronary optical coherence tomography (OCT) images to better guide the treatment of coronary artery disease. However, existing methods either require a large pixel-wisely paired training dataset or have limited capability to map pathological regions. To address these issues, we proposed a structural constrained, pathology aware, transformer generative adversarial network, namely SCPAT-GAN, to generate virtual stained H&E histology from OCT images. The proposed SCPAT-GAN advances existing methods via a novel design to impose pathological guidance on structural layers using transformer-based network.
Structural constrained virtual histology staining for human coronary imaging using deep learning
Nov 12, 2022



Abstract:Histopathological analysis is crucial in artery characterization for coronary artery disease (CAD). However, histology requires an invasive and time-consuming process. In this paper, we propose to generate virtual histology staining using Optical Coherence Tomography (OCT) images to enable real-time histological visualization. We develop a deep learning network, namely Coronary-GAN, to transfer coronary OCT images to virtual histology images. With a special consideration on the structural constraints in coronary OCT images, our method achieves better image generation performance than the conventional GAN-based method. The experimental results indicate that Coronary-GAN generates virtual histology images that are similar to real histology images, revealing the human coronary layers.
Towards reliable calcification detection: calibration of uncertainty in coronary optical coherence tomography images
Nov 12, 2022Abstract:Optical coherence tomography (OCT) has become increasingly essential in assisting the treatment of coronary artery disease (CAD). Image-guided solutions such as Percutaneous Coronary Intervention (PCI) are extensively used during the treatment of CAD. However, unidentified calcified regions within a narrowed artery could impair the outcome of the PCI. Prior to treatments, object detection is paramount to automatically procure accurate readings on the location and thickness of calcifications within the artery. Deep learning-based object detection methods have been explored in a variety of applications. The quality of object detection predictions could lead to uncertain results, which are not desirable in safety-critical scenarios. In this work, we implement an object detection model, You-Only-Look-Once v5 (YOLO), on a calcification detection framework within coronary OCT images. We evaluate the uncertainty of predictions based on the expected calibration errors, thus assessing the certainty level of detection results. To calibrate confidence scores of predictions, we implement dependent logistic calibration using each detection result's confidence and center coordinates. With the calibrated confidence score of each prediction, we lower the uncertainty of predictions in calcification detection. Our results show that the YOLO achieves higher precision and recall in comparison with the other object detection model, meanwhile producing more reliable results. The calibrated confidence of prediction results in a confidence error of approximately 0.13, suggesting that the confidence calibration on calcification detection could provide a more trustworthy result, indicating a great potential to assist clinical evaluation of treating the CAD during the imaging-guided procedure.
Multi-scale reconstruction of undersampled spectral-spatial OCT data for coronary imaging using deep learning
Apr 25, 2022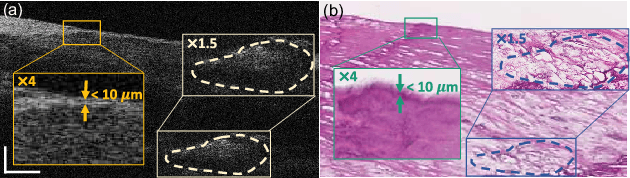
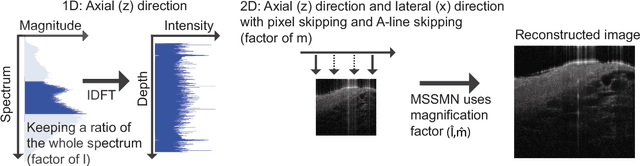
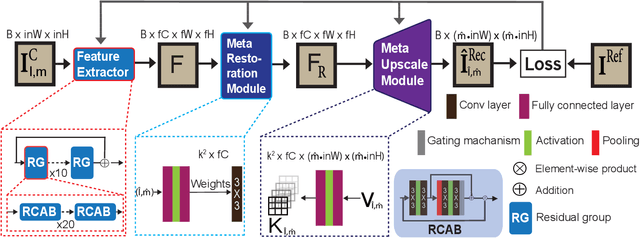

Abstract:Coronary artery disease (CAD) is a cardiovascular condition with high morbidity and mortality. Intravascular optical coherence tomography (IVOCT) has been considered as an optimal imagining system for the diagnosis and treatment of CAD. Constrained by Nyquist theorem, dense sampling in IVOCT attains high resolving power to delineate cellular structures/ features. There is a trade-off between high spatial resolution and fast scanning rate for coronary imaging. In this paper, we propose a viable spectral-spatial acquisition method that down-scales the sampling process in both spectral and spatial domain while maintaining high quality in image reconstruction. The down-scaling schedule boosts data acquisition speed without any hardware modifications. Additionally, we propose a unified multi-scale reconstruction framework, namely Multiscale- Spectral-Spatial-Magnification Network (MSSMN), to resolve highly down-scaled (compressed) OCT images with flexible magnification factors. We incorporate the proposed methods into Spectral Domain OCT (SD-OCT) imaging of human coronary samples with clinical features such as stent and calcified lesions. Our experimental results demonstrate that spectral-spatial downscaled data can be better reconstructed than data that is downscaled solely in either spectral or spatial domain. Moreover, we observe better reconstruction performance using MSSMN than using existing reconstruction methods. Our acquisition method and multi-scale reconstruction framework, in combination, may allow faster SD-OCT inspection with high resolution during coronary intervention.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge