Shovito Barua Soumma
Counterfactual Modeling with Fine-Tuned LLMs for Health Intervention Design and Sensor Data Augmentation
Jan 21, 2026Abstract:Counterfactual explanations (CFEs) provide human-centric interpretability by identifying the minimal, actionable changes required to alter a machine learning model's prediction. Therefore, CFs can be used as (i) interventions for abnormality prevention and (ii) augmented data for training robust models. We conduct a comprehensive evaluation of CF generation using large language models (LLMs), including GPT-4 (zero-shot and few-shot) and two open-source models-BioMistral-7B and LLaMA-3.1-8B, in both pretrained and fine-tuned configurations. Using the multimodal AI-READI clinical dataset, we assess CFs across three dimensions: intervention quality, feature diversity, and augmentation effectiveness. Fine-tuned LLMs, particularly LLaMA-3.1-8B, produce CFs with high plausibility (up to 99%), strong validity (up to 0.99), and realistic, behaviorally modifiable feature adjustments. When used for data augmentation under controlled label-scarcity settings, LLM-generated CFs substantially restore classifier performance, yielding an average 20% F1 recovery across three scarcity scenarios. Compared with optimization-based baselines such as DiCE, CFNOW, and NICE, LLMs offer a flexible, model-agnostic approach that generates more clinically actionable and semantically coherent counterfactuals. Overall, this work demonstrates the promise of LLM-driven counterfactuals for both interpretable intervention design and data-efficient model training in sensor-based digital health. Impact: SenseCF fine-tunes an LLM to generate valid, representative counterfactual explanations and supplement minority class in an imbalanced dataset for improving model training and boosting model robustness and predictive performance
Enhancing Metabolic Syndrome Prediction with Hybrid Data Balancing and Counterfactuals
Apr 09, 2025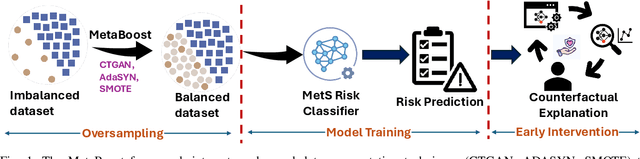

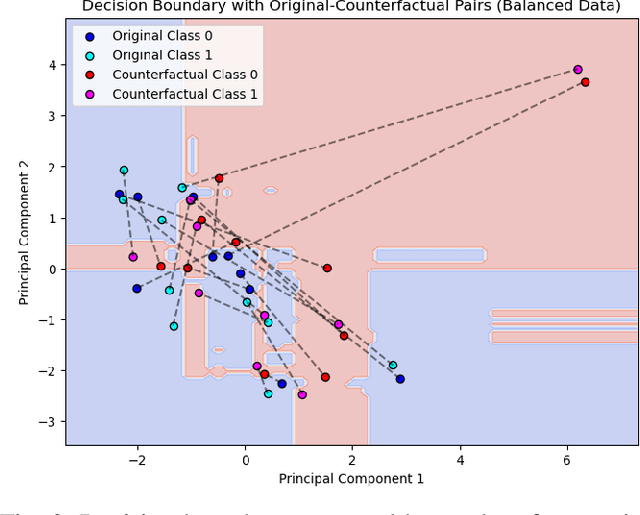

Abstract:Metabolic Syndrome (MetS) is a cluster of interrelated risk factors that significantly increases the risk of cardiovascular diseases and type 2 diabetes. Despite its global prevalence, accurate prediction of MetS remains challenging due to issues such as class imbalance, data scarcity, and methodological inconsistencies in existing studies. In this paper, we address these challenges by systematically evaluating and optimizing machine learning (ML) models for MetS prediction, leveraging advanced data balancing techniques and counterfactual analysis. Multiple ML models, including XGBoost, Random Forest, TabNet, etc., were trained and compared under various data balancing techniques such as random oversampling (ROS), SMOTE, ADASYN, and CTGAN. Additionally, we introduce MetaBoost, a novel hybrid framework that integrates SMOTE, ADASYN, and CTGAN, optimizing synthetic data generation through weighted averaging and iterative weight tuning to enhance the model's performance (achieving a 1.14% accuracy improvement over individual balancing techniques). A comprehensive counterfactual analysis is conducted to quantify feature-level changes required to shift individuals from high-risk to low-risk categories. The results indicate that blood glucose (50.3%) and triglycerides (46.7%) were the most frequently modified features, highlighting their clinical significance in MetS risk reduction. Additionally, probabilistic analysis shows elevated blood glucose (85.5% likelihood) and triglycerides (74.9% posterior probability) as the strongest predictors. This study not only advances the methodological rigor of MetS prediction but also provides actionable insights for clinicians and researchers, highlighting the potential of ML in mitigating the public health burden of metabolic syndrome.
Design and Implementation of a Scalable Clinical Data Warehouse for Resource-Constrained Healthcare Systems
Feb 23, 2025Abstract:Centralized electronic health record repositories are critical for advancing disease surveillance, public health research, and evidence-based policymaking. However, developing countries face persistent challenges in achieving this due to fragmented healthcare data sources, inconsistent record-keeping practices, and the absence of standardized patient identifiers, limiting reliable record linkage, compromise data interoperability, and limit scalability-obstacles exacerbated by infrastructural constraints and privacy concerns. To address these barriers, this study proposes a scalable, privacy-preserving clinical data warehouse, NCDW, designed for heterogeneous EHR integration in resource-limited settings and tested with 1.16 million clinical records. The framework incorporates a wrapper-based data acquisition layer for secure, automated ingestion of multisource health data and introduces a soundex algorithm to resolve patient identity mismatches in the absence of unique IDs. A modular data mart is designed for disease-specific analytics, demonstrated through a dengue fever case study in Bangladesh, integrating clinical, demographic, and environmental data for outbreak prediction and resource planning. Quantitative assessment of the data mart underscores its utility in strengthening national decision-support systems, highlighting the model's adaptability for infectious disease management. Comparative evaluation of database technologies reveals NoSQL outperforms relational SQL by 40-69% in complex query processing, while system load estimates validate the architecture's capacity to manage 19 million daily records (34TB over 5 years). The framework can be adapted to various healthcare settings across developing nations by modifying the ingestion layer to accommodate standards like ICD-11 and HL7 FHIR, facilitating interoperability for managing infectious diseases (i.e., COVID, tuberculosis).
Freezing of Gait Detection Using Gramian Angular Fields and Federated Learning from Wearable Sensors
Nov 19, 2024
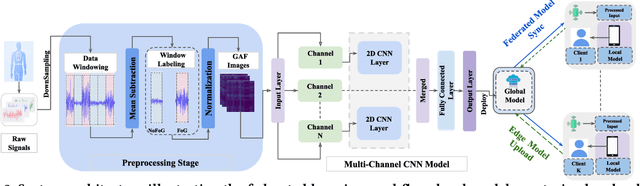

Abstract:Freezing of gait (FOG) is a debilitating symptom of Parkinson's disease (PD) that impairs mobility and safety. Traditional detection methods face challenges due to intra and inter-patient variability, and most systems are tested in controlled settings, limiting their real-world applicability. Addressing these gaps, we present FOGSense, a novel FOG detection system designed for uncontrolled, free-living conditions. It uses Gramian Angular Field (GAF) transformations and federated deep learning to capture temporal and spatial gait patterns missed by traditional methods. We evaluated our FOGSense system using a public PD dataset, 'tdcsfog'. FOGSense improves accuracy by 10.4% over a single-axis accelerometer, reduces failure points compared to multi-sensor systems, and demonstrates robustness to missing values. The federated architecture allows personalized model adaptation and efficient smartphone synchronization during off-peak hours, making it effective for long-term monitoring as symptoms evolve. Overall, FOGSense achieves a 22.2% improvement in F1-score compared to state-of-the-art methods, along with enhanced sensitivity for FOG episode detection. Code is available: https://github.com/shovito66/FOGSense.
Hybrid Attention Model Using Feature Decomposition and Knowledge Distillation for Glucose Forecasting
Nov 16, 2024Abstract:The availability of continuous glucose monitors as over-the-counter commodities have created a unique opportunity to monitor a person's blood glucose levels, forecast blood glucose trajectories and provide automated interventions to prevent devastating chronic complications that arise from poor glucose control. However, forecasting blood glucose levels is challenging because blood glucose changes consistently in response to food intake, medication intake, physical activity, sleep, and stress. It is particularly difficult to accurately predict BGL from multimodal and irregularly sampled data and over long prediction horizons. Furthermore, these forecasting models must operate in real-time on edge devices to provide in-the-moment interventions. To address these challenges, we propose GlucoNet, an AI-powered sensor system for continuously monitoring behavioral and physiological health and robust forecasting of blood glucose patterns. GlucoNet devises a feature decomposition-based transformer model that incorporates patients' behavioral and physiological data and transforms sparse and irregular patient data (e.g., diet and medication intake data) into continuous features using a mathematical model, facilitating better integration with the BGL data. Given the non-linear and non-stationary nature of BG signals, we propose a decomposition method to extract both low and high-frequency components from the BGL signals, thus providing accurate forecasting. To reduce the computational complexity, we also propose to employ knowledge distillation to compress the transformer model. GlucoNet achieves a 60% improvement in RMSE and a 21% reduction in the number of parameters, using data obtained involving 12 participants with T1-Diabetes. These results underscore GlucoNet's potential as a compact and reliable tool for real-world diabetes prevention and management.
Efficient Feature Extraction and Classification Architecture for MRI-Based Brain Tumor Detection
Oct 30, 2024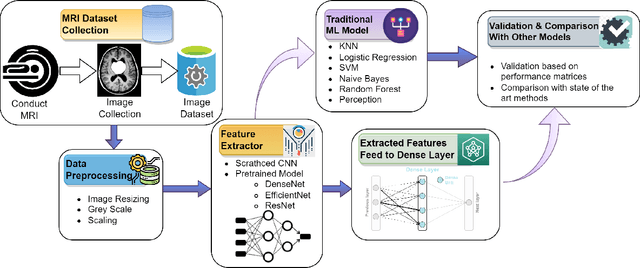
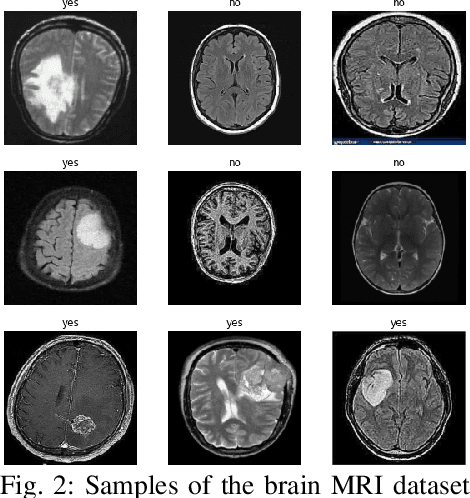
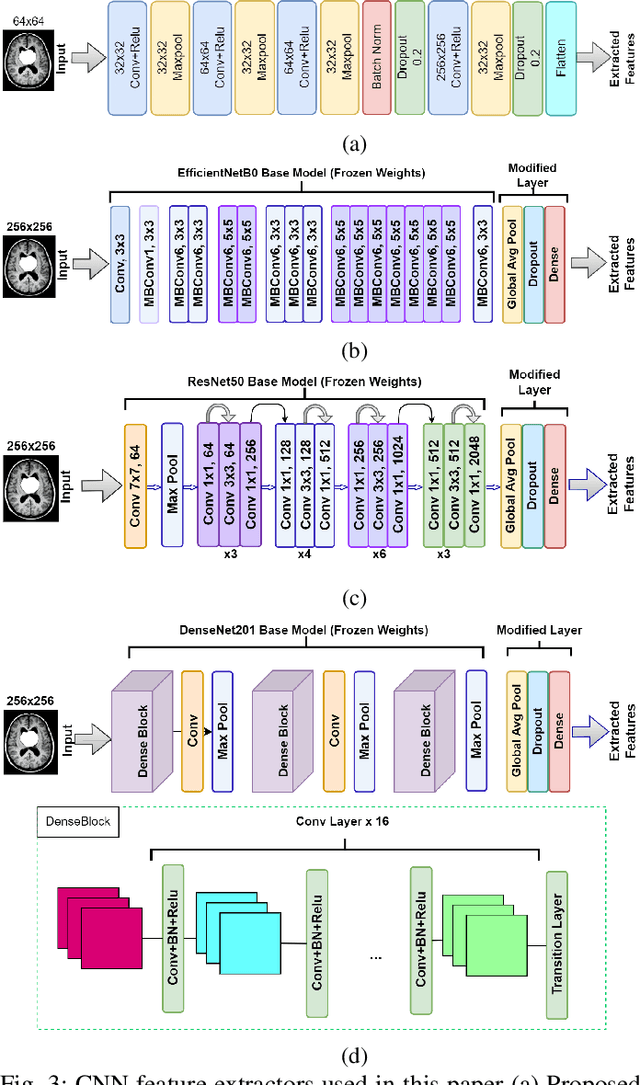
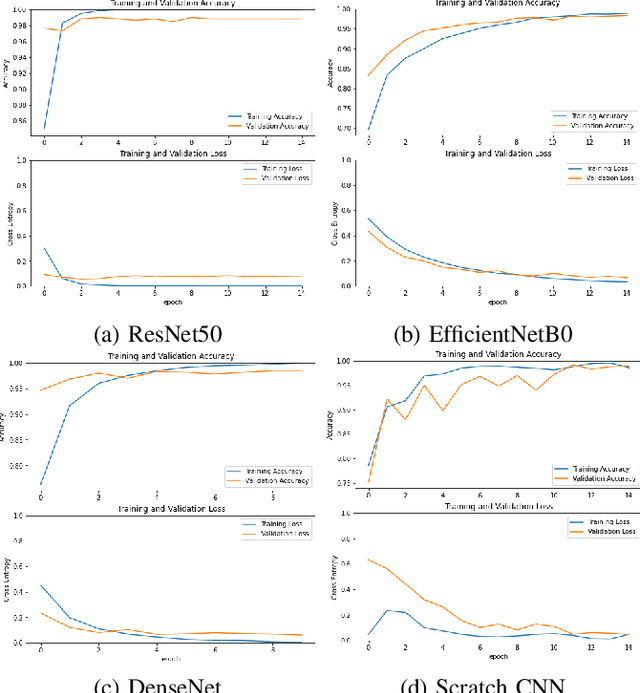
Abstract:Uncontrolled cell division in the brain is what gives rise to brain tumors. If the tumor size increases by more than half, there is little hope for the patient's recovery. This emphasizes the need of rapid and precise brain tumor diagnosis. When it comes to analyzing, diagnosing, and planning therapy for brain tumors, MRI imaging plays a crucial role. A brain tumor's development history is crucial information for doctors to have. When it comes to distinguishing between human soft tissues, MRI scans are superior. In order to get reliable classification results from MRI scans quickly, deep learning is one of the most practical methods. Early human illness diagnosis has been demonstrated to be more accurate when deep learning methods are used. In the case of diagnosing a brain tumor, when even a little misdiagnosis might have serious consequences, accuracy is especially important. Disclosure of brain tumors in medical images is still a difficult task. Brain MRIs are notoriously imprecise in revealing the presence or absence of tumors. Using MRI scans of the brain, a Convolutional Neural Network (CNN) was trained to identify the presence of a tumor in this research. Results from the CNN model showed an accuracy of 99.17%. The CNN model's characteristics were also retrieved. In order to evaluate the CNN model's capability for processing images, we applied the features via the following machine learning models: KNN, Logistic regression, SVM, Random Forest, Naive Bayes, and Perception. CNN and machine learning models were also evaluated using the standard metrics of Precision, Recall, Specificity, and F1 score. The significance of the doctor's diagnosis enhanced the accuracy of the CNN model's assistance in identifying the existence of tumor and treating the patient.
Self-Supervised Learning and Opportunistic Inference for Continuous Monitoring of Freezing of Gait in Parkinson's Disease
Oct 27, 2024



Abstract:Parkinson's disease (PD) is a progressive neurological disorder that impacts the quality of life significantly, making in-home monitoring of motor symptoms such as Freezing of Gait (FoG) critical. However, existing symptom monitoring technologies are power-hungry, rely on extensive amounts of labeled data, and operate in controlled settings. These shortcomings limit real-world deployment of the technology. This work presents LIFT-PD, a computationally-efficient self-supervised learning framework for real-time FoG detection. Our method combines self-supervised pre-training on unlabeled data with a novel differential hopping windowing technique to learn from limited labeled instances. An opportunistic model activation module further minimizes power consumption by selectively activating the deep learning module only during active periods. Extensive experimental results show that LIFT-PD achieves a 7.25% increase in precision and 4.4% improvement in accuracy compared to supervised models while using as low as 40% of the labeled training data used for supervised learning. Additionally, the model activation module reduces inference time by up to 67% compared to continuous inference. LIFT-PD paves the way for practical, energy-efficient, and unobtrusive in-home monitoring of PD patients with minimal labeling requirements.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge