Seiji Okada
3DDX: Bone Surface Reconstruction from a Single Standard-Geometry Radiograph via Dual-Face Depth Estimation
Sep 25, 2024Abstract:Radiography is widely used in orthopedics for its affordability and low radiation exposure. 3D reconstruction from a single radiograph, so-called 2D-3D reconstruction, offers the possibility of various clinical applications, but achieving clinically viable accuracy and computational efficiency is still an unsolved challenge. Unlike other areas in computer vision, X-ray imaging's unique properties, such as ray penetration and fixed geometry, have not been fully exploited. We propose a novel approach that simultaneously learns multiple depth maps (front- and back-surface of multiple bones) derived from the X-ray image to computed tomography registration. The proposed method not only leverages the fixed geometry characteristic of X-ray imaging but also enhances the precision of the reconstruction of the whole surface. Our study involved 600 CT and 2651 X-ray images (4 to 5 posed X-ray images per patient), demonstrating our method's superiority over traditional approaches with a surface reconstruction error reduction from 4.78 mm to 1.96 mm. This significant accuracy improvement and enhanced computational efficiency suggest our approach's potential for clinical application.
Validation of musculoskeletal segmentation model with uncertainty estimation for bone and muscle assessment in hip-to-knee clinical CT images
Sep 04, 2024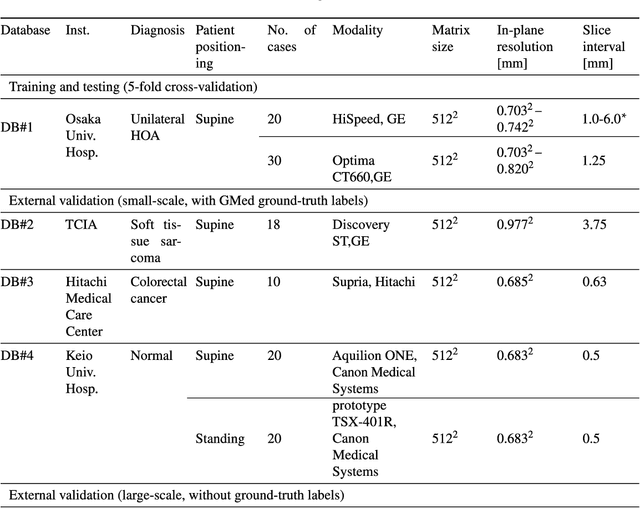

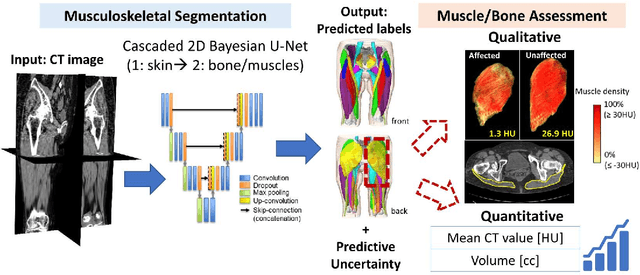
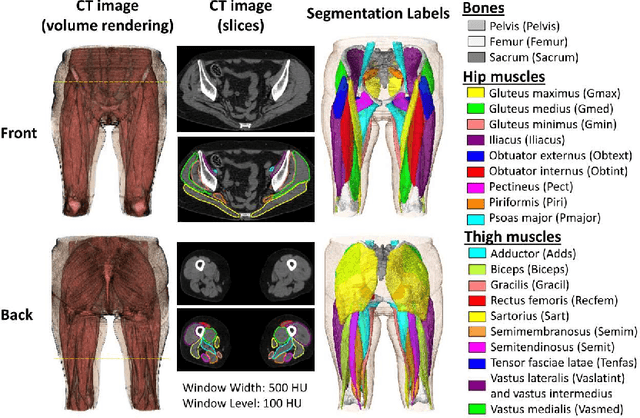
Abstract:Deep learning-based image segmentation has allowed for the fully automated, accurate, and rapid analysis of musculoskeletal (MSK) structures from medical images. However, current approaches were either applied only to 2D cross-sectional images, addressed few structures, or were validated on small datasets, which limit the application in large-scale databases. This study aimed to validate an improved deep learning model for volumetric MSK segmentation of the hip and thigh with uncertainty estimation from clinical computed tomography (CT) images. Databases of CT images from multiple manufacturers/scanners, disease status, and patient positioning were used. The segmentation accuracy, and accuracy in estimating the structures volume and density, i.e., mean HU, were evaluated. An approach for segmentation failure detection based on predictive uncertainty was also investigated. The model has shown an overall improvement with respect to all segmentation accuracy and structure volume/density evaluation metrics. The predictive uncertainty yielded large areas under the receiver operating characteristic (AUROC) curves (AUROCs>=.95) in detecting inaccurate and failed segmentations. The high segmentation and muscle volume/density estimation accuracy, along with the high accuracy in failure detection based on the predictive uncertainty, exhibited the model's reliability for analyzing individual MSK structures in large-scale CT databases.
Enhancing Quantitative Image Synthesis through Pretraining and Resolution Scaling for Bone Mineral Density Estimation from a Plain X-ray Image
Jul 30, 2024Abstract:While most vision tasks are essentially visual in nature (for recognition), some important tasks, especially in the medical field, also require quantitative analysis (for quantification) using quantitative images. Unlike in visual analysis, pixel values in quantitative images correspond to physical metrics measured by specific devices (e.g., a depth image). However, recent work has shown that it is sometimes possible to synthesize accurate quantitative values from visual ones (e.g., depth from visual cues or defocus). This research aims to improve quantitative image synthesis (QIS) by exploring pretraining and image resolution scaling. We propose a benchmark for evaluating pretraining performance using the task of QIS-based bone mineral density (BMD) estimation from plain X-ray images, where the synthesized quantitative image is used to derive BMD. Our results show that appropriate pretraining can improve QIS performance, significantly raising the correlation of BMD estimation from 0.820 to 0.898, while others do not help or even hinder it. Scaling-up the resolution can further boost the correlation up to 0.923, a significant enhancement over conventional methods. Future work will include exploring more pretraining strategies and validating them on other image synthesis tasks.
Automatic hip osteoarthritis grading with uncertainty estimation from computed tomography using digitally-reconstructed radiographs
Dec 30, 2023Abstract:Progression of hip osteoarthritis (hip OA) leads to pain and disability, likely leading to surgical treatment such as hip arthroplasty at the terminal stage. The severity of hip OA is often classified using the Crowe and Kellgren-Lawrence (KL) classifications. However, as the classification is subjective, we aimed to develop an automated approach to classify the disease severity based on the two grades using digitally-reconstructed radiographs (DRRs) from CT images. Automatic grading of the hip OA severity was performed using deep learning-based models. The models were trained to predict the disease grade using two grading schemes, i.e., predicting the Crowe and KL grades separately, and predicting a new ordinal label combining both grades and representing the disease progression of hip OA. The models were trained in classification and regression settings. In addition, the model uncertainty was estimated and validated as a predictor of classification accuracy. The models were trained and validated on a database of 197 hip OA patients, and externally validated on 52 patients. The model accuracy was evaluated using exact class accuracy (ECA), one-neighbor class accuracy (ONCA), and balanced accuracy.The deep learning models produced a comparable accuracy of approximately 0.65 (ECA) and 0.95 (ONCA) in the classification and regression settings. The model uncertainty was significantly larger in cases with large classification errors (P<6e-3). In this study, an automatic approach for grading hip OA severity from CT images was developed. The models have shown comparable performance with high ONCA, which facilitates automated grading in large-scale CT databases and indicates the potential for further disease progression analysis. Classification accuracy was correlated with the model uncertainty, which would allow for the prediction of classification errors.
Bone mineral density estimation from a plain X-ray image by learning decomposition into projections of bone-segmented computed tomography
Jul 21, 2023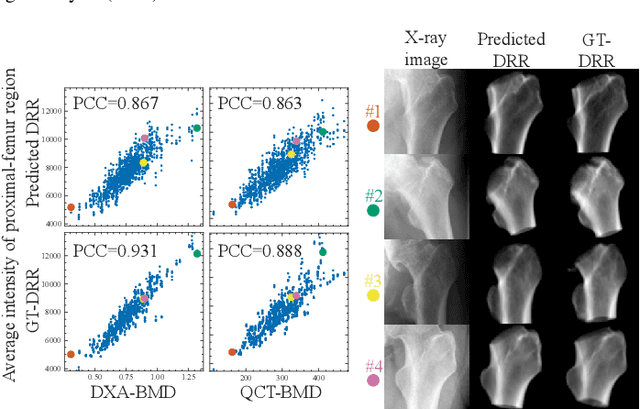
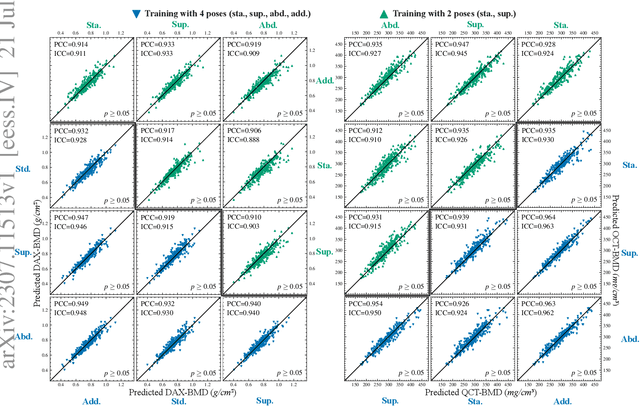
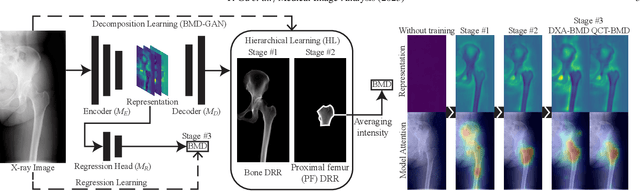
Abstract:Osteoporosis is a prevalent bone disease that causes fractures in fragile bones, leading to a decline in daily living activities. Dual-energy X-ray absorptiometry (DXA) and quantitative computed tomography (QCT) are highly accurate for diagnosing osteoporosis; however, these modalities require special equipment and scan protocols. To frequently monitor bone health, low-cost, low-dose, and ubiquitously available diagnostic methods are highly anticipated. In this study, we aim to perform bone mineral density (BMD) estimation from a plain X-ray image for opportunistic screening, which is potentially useful for early diagnosis. Existing methods have used multi-stage approaches consisting of extraction of the region of interest and simple regression to estimate BMD, which require a large amount of training data. Therefore, we propose an efficient method that learns decomposition into projections of bone-segmented QCT for BMD estimation under limited datasets. The proposed method achieved high accuracy in BMD estimation, where Pearson correlation coefficients of 0.880 and 0.920 were observed for DXA-measured BMD and QCT-measured BMD estimation tasks, respectively, and the root mean square of the coefficient of variation values were 3.27 to 3.79% for four measurements with different poses. Furthermore, we conducted extensive validation experiments, including multi-pose, uncalibrated-CT, and compression experiments toward actual application in routine clinical practice.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge