Sandra Camelo-Piragua
Artificial-intelligence-based molecular classification of diffuse gliomas using rapid, label-free optical imaging
Mar 23, 2023Abstract:Molecular classification has transformed the management of brain tumors by enabling more accurate prognostication and personalized treatment. However, timely molecular diagnostic testing for patients with brain tumors is limited, complicating surgical and adjuvant treatment and obstructing clinical trial enrollment. In this study, we developed DeepGlioma, a rapid ($< 90$ seconds), artificial-intelligence-based diagnostic screening system to streamline the molecular diagnosis of diffuse gliomas. DeepGlioma is trained using a multimodal dataset that includes stimulated Raman histology (SRH); a rapid, label-free, non-consumptive, optical imaging method; and large-scale, public genomic data. In a prospective, multicenter, international testing cohort of patients with diffuse glioma ($n=153$) who underwent real-time SRH imaging, we demonstrate that DeepGlioma can predict the molecular alterations used by the World Health Organization to define the adult-type diffuse glioma taxonomy (IDH mutation, 1p19q co-deletion and ATRX mutation), achieving a mean molecular classification accuracy of $93.3\pm 1.6\%$. Our results represent how artificial intelligence and optical histology can be used to provide a rapid and scalable adjunct to wet lab methods for the molecular screening of patients with diffuse glioma.
OpenSRH: optimizing brain tumor surgery using intraoperative stimulated Raman histology
Jun 16, 2022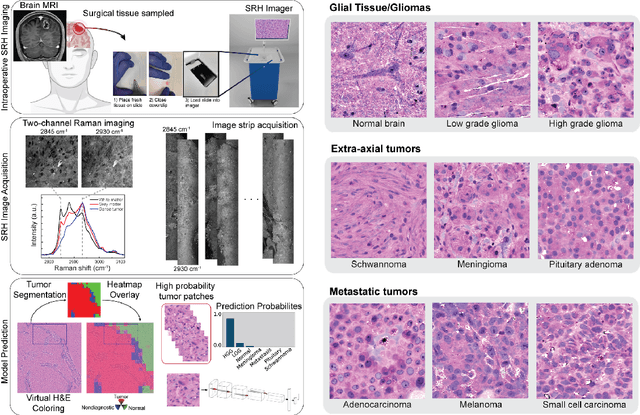
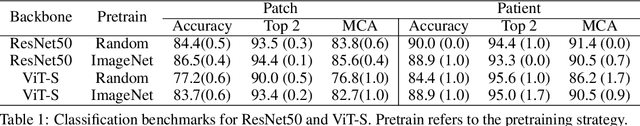
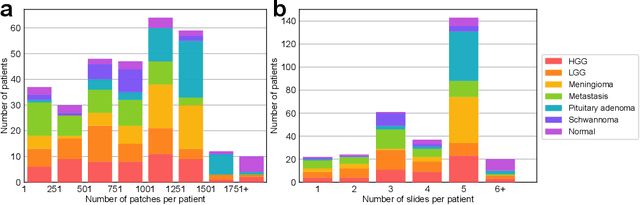
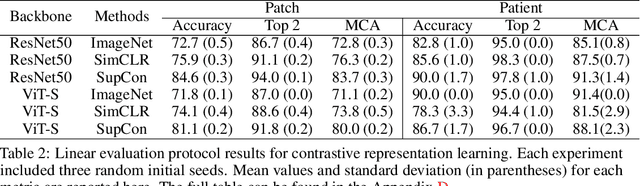
Abstract:Accurate intraoperative diagnosis is essential for providing safe and effective care during brain tumor surgery. Our standard-of-care diagnostic methods are time, resource, and labor intensive, which restricts access to optimal surgical treatments. To address these limitations, we propose an alternative workflow that combines stimulated Raman histology (SRH), a rapid optical imaging method, with deep learning-based automated interpretation of SRH images for intraoperative brain tumor diagnosis and real-time surgical decision support. Here, we present OpenSRH, the first public dataset of clinical SRH images from 300+ brain tumors patients and 1300+ unique whole slide optical images. OpenSRH contains data from the most common brain tumors diagnoses, full pathologic annotations, whole slide tumor segmentations, raw and processed optical imaging data for end-to-end model development and validation. We provide a framework for patch-based whole slide SRH classification and inference using weak (i.e. patient-level) diagnostic labels. Finally, we benchmark two computer vision tasks: multiclass histologic brain tumor classification and patch-based contrastive representation learning. We hope OpenSRH will facilitate the clinical translation of rapid optical imaging and real-time ML-based surgical decision support in order to improve the access, safety, and efficacy of cancer surgery in the era of precision medicine. Dataset access, code, and benchmarks are available at opensrh.mlins.org.
Contrastive Representation Learning for Rapid Intraoperative Diagnosis of Skull Base Tumors Imaged Using Stimulated Raman Histology
Aug 08, 2021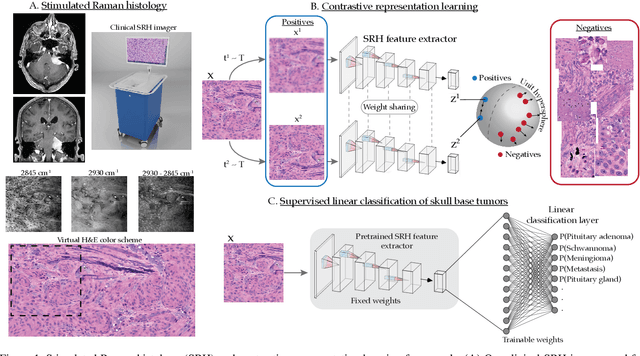
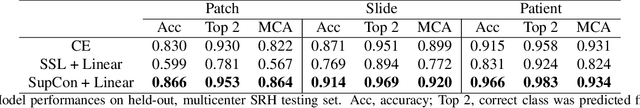
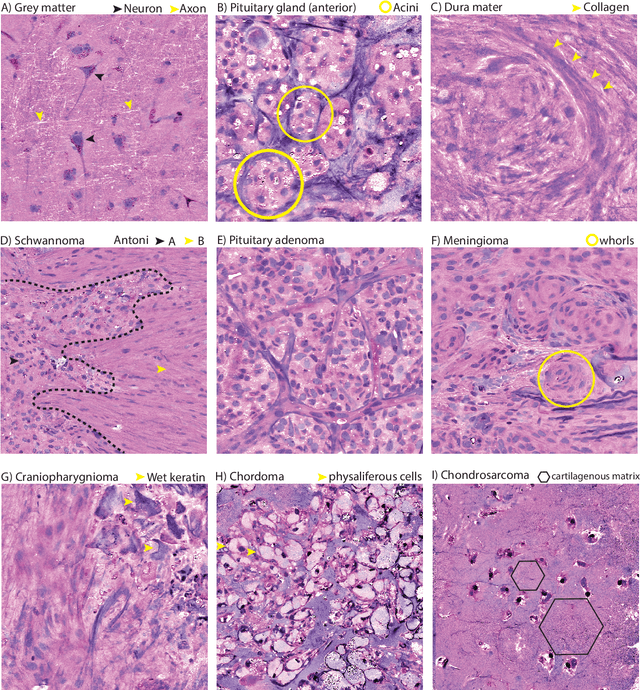
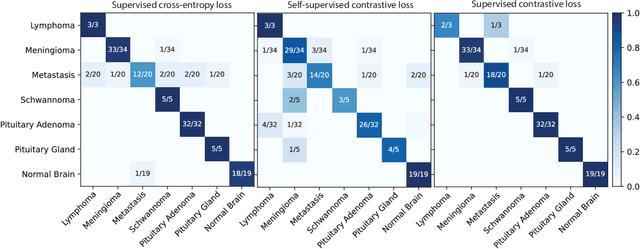
Abstract:Background: Accurate diagnosis of skull base tumors is essential for providing personalized surgical treatment strategies. Intraoperative diagnosis can be challenging due to tumor diversity and lack of intraoperative pathology resources. Objective: To develop an independent and parallel intraoperative pathology workflow that can provide rapid and accurate skull base tumor diagnoses using label-free optical imaging and artificial intelligence (AI). Method: We used a fiber laser-based, label-free, non-consumptive, high-resolution microscopy method ($<$ 60 sec per 1 $\times$ 1 mm$^\text{2}$), called stimulated Raman histology (SRH), to image a consecutive, multicenter cohort of skull base tumor patients. SRH images were then used to train a convolutional neural network (CNN) model using three representation learning strategies: cross-entropy, self-supervised contrastive learning, and supervised contrastive learning. Our trained CNN models were tested on a held-out, multicenter SRH dataset. Results: SRH was able to image the diagnostic features of both benign and malignant skull base tumors. Of the three representation learning strategies, supervised contrastive learning most effectively learned the distinctive and diagnostic SRH image features for each of the skull base tumor types. In our multicenter testing set, cross-entropy achieved an overall diagnostic accuracy of 91.5%, self-supervised contrastive learning 83.9%, and supervised contrastive learning 96.6%. Our trained model was able to identify tumor-normal margins and detect regions of microscopic tumor infiltration in whole-slide SRH images. Conclusion: SRH with AI models trained using contrastive representation learning can provide rapid and accurate intraoperative diagnosis of skull base tumors.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge