Sadahiro Kaneko
A Spectral Library and Method for Sparse Unmixing of Hyperspectral Images in Fluorescence Guided Resection of Brain Tumors
Jan 30, 2024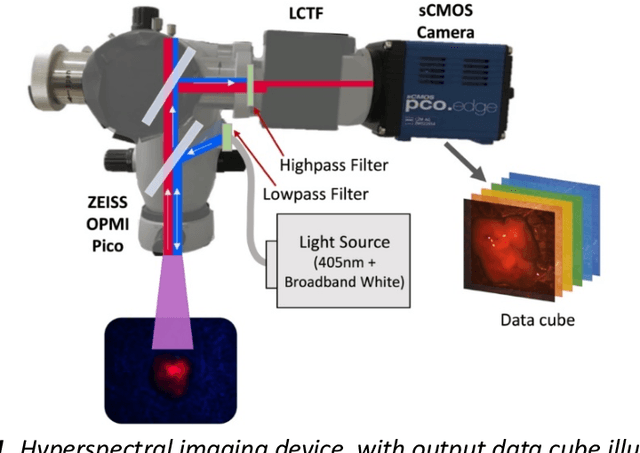
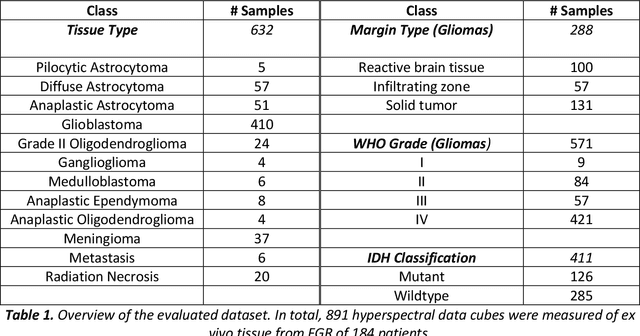
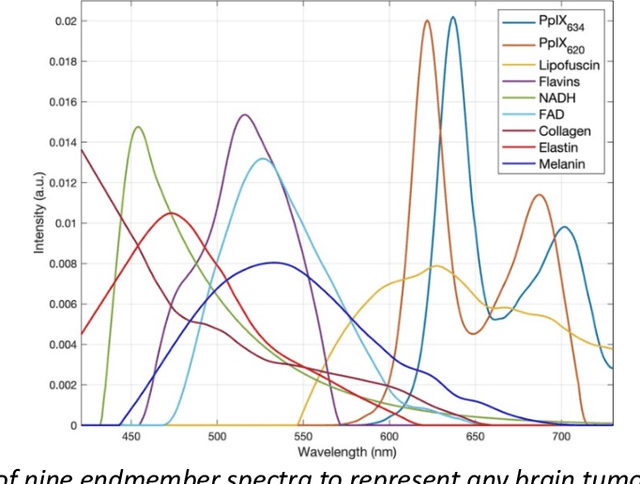
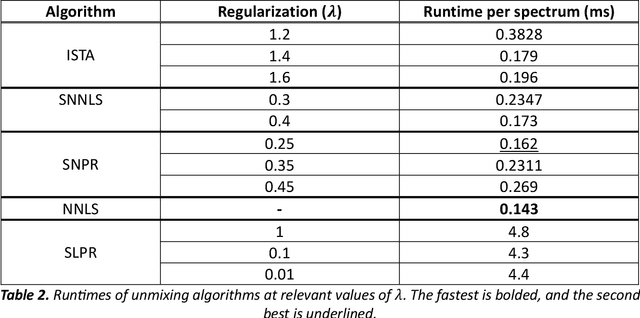
Abstract:Through spectral unmixing, hyperspectral imaging (HSI) in fluorescence-guided brain tumor surgery has enabled detection and classification of tumor regions invisible to the human eye. Prior unmixing work has focused on determining a minimal set of viable fluorophore spectra known to be present in the brain and effectively reconstructing human data without overfitting. With these endmembers, non-negative least squares regression (NNLS) was used to compute the abundances. However, HSI images are heterogeneous, so one small set of endmember spectra may not fit all pixels well. Additionally, NNLS is the maximum likelihood estimator only if the measurement is normally distributed, and it does not enforce sparsity, which leads to overfitting and unphysical results. Here, we analyzed 555666 HSI fluorescence spectra from 891 ex vivo measurements of patients with brain tumors to show that a Poisson distribution models the measured data 82% better than a Gaussian in terms of the Kullback-Leibler divergence and that the endmember abundance vectors are sparse. With this knowledge, we introduce (1) a library of 9 endmember spectra, (2) a sparse, non-negative Poisson regression algorithm to perform physics-informed unmixing with this library without overfitting, and (3) a highly realistic spectral measurement simulation with known endmember abundances. The new unmixing method was then tested on the human and simulated data and compared to four other candidate methods. It outperforms previous methods with 25% lower error in the computed abundances on the simulated data than NNLS, lower reconstruction error on human data, beUer sparsity, and 31 times faster runtime than state-of-the-art Poisson regression. This method and library of endmember spectra can enable more accurate spectral unmixing to beUer aid the surgeon during brain tumor resection.
Towards Machine Learning-based Quantitative Hyperspectral Image Guidance for Brain Tumor Resection
Nov 24, 2023Abstract:Complete resection of malignant gliomas is hampered by the difficulty in distinguishing tumor cells at the infiltration zone. Fluorescence guidance with 5-ALA assists in reaching this goal. Using hyperspectral imaging, previous work characterized five fluorophores' emission spectra in most human brain tumors. In this paper, the effectiveness of these five spectra was explored for different tumor and tissue classification tasks in 184 patients (891 hyperspectral measurements) harboring low- (n=30) and high-grade gliomas (n=115), non-glial primary brain tumors (n=19), radiation necrosis (n=2), miscellaneous (n=10) and metastases (n=8). Four machine learning models were trained to classify tumor type, grade, glioma margins and IDH mutation. Using random forests and multi-layer perceptrons, the classifiers achieved average test accuracies of 84-87%, 96%, 86%, and 93% respectively. All five fluorophore abundances varied between tumor margin types and tumor grades (p < 0.01). For tissue type, at least four of the five fluorophore abundances were found to be significantly different (p < 0.01) between all classes. These results demonstrate the fluorophores' differing abundances in different tissue classes, as well as the value of the five fluorophores as potential optical biomarkers, opening new opportunities for intraoperative classification systems in fluorescence-guided neurosurgery.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge