Rushi Jiao
Segment Anything Model for Medical Image Segmentation: Current Applications and Future Directions
Jan 07, 2024Abstract:Due to the inherent flexibility of prompting, foundation models have emerged as the predominant force in the fields of natural language processing and computer vision. The recent introduction of the Segment Anything Model (SAM) signifies a noteworthy expansion of the prompt-driven paradigm into the domain of image segmentation, thereby introducing a plethora of previously unexplored capabilities. However, the viability of its application to medical image segmentation remains uncertain, given the substantial distinctions between natural and medical images. In this work, we provide a comprehensive overview of recent endeavors aimed at extending the efficacy of SAM to medical image segmentation tasks, encompassing both empirical benchmarking and methodological adaptations. Additionally, we explore potential avenues for future research directions in SAM's role within medical image segmentation. While direct application of SAM to medical image segmentation does not yield satisfactory performance on multi-modal and multi-target medical datasets so far, numerous insights gleaned from these efforts serve as valuable guidance for shaping the trajectory of foundational models in the realm of medical image analysis. To support ongoing research endeavors, we maintain an active repository that contains an up-to-date paper list and a succinct summary of open-source projects at https://github.com/YichiZhang98/SAM4MIS.
How Segment Anything Model (SAM) Boost Medical Image Segmentation?
May 05, 2023Abstract:Due to the flexibility of prompting, foundation models have become the dominant force in the domains of natural language processing and image generation. With the recent introduction of the Segment Anything Model (SAM), the prompt-driven paradigm has entered the realm of image segmentation, bringing with a range of previously unexplored capabilities. However, it remains unclear whether it can be applicable to medical image segmentation due to the significant differences between natural images and medical images. In this report, we summarize recent efforts to extend the success of SAM to medical image segmentation tasks, including both empirical benchmarking and methodological adaptations, and discuss potential future directions for SAM in medical image segmentation. We also set up a collection of literature reviews to boost the research on this topic at https://github.com/YichiZhang98/SAM4MIS.
Learning with Limited Annotations: A Survey on Deep Semi-Supervised Learning for Medical Image Segmentation
Aug 13, 2022
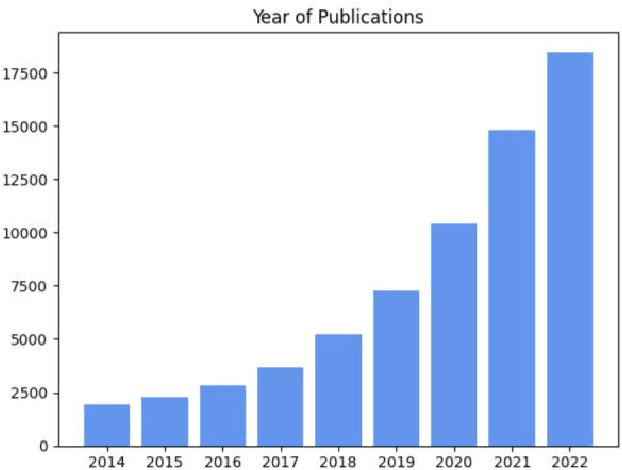
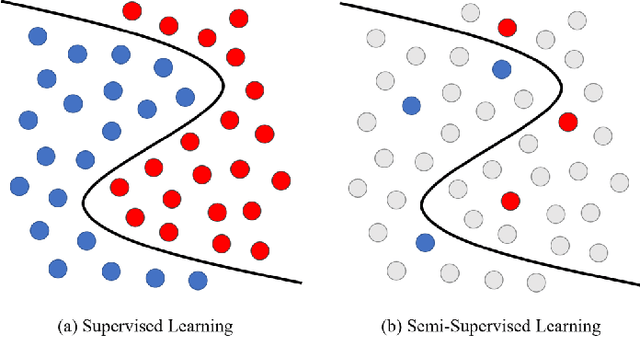
Abstract:Medical image segmentation is a fundamental and critical step in many image-guided clinical approaches. Recent success of deep learning-based segmentation methods usually relies on a large amount of labeled data, which is particularly difficult and costly to obtain especially in the medical imaging domain where only experts can provide reliable and accurate annotations. Semi-supervised learning has emerged as an appealing strategy and been widely applied to medical image segmentation tasks to train deep models with limited annotations. In this paper, we present a comprehensive review of recently proposed semi-supervised learning methods for medical image segmentation and summarized both the technical novelties and empirical results. Furthermore, we analyze and discuss the limitations and several unsolved problems of existing approaches. We hope this review could inspire the research community to explore solutions for this challenge and further promote the developments in medical image segmentation field.
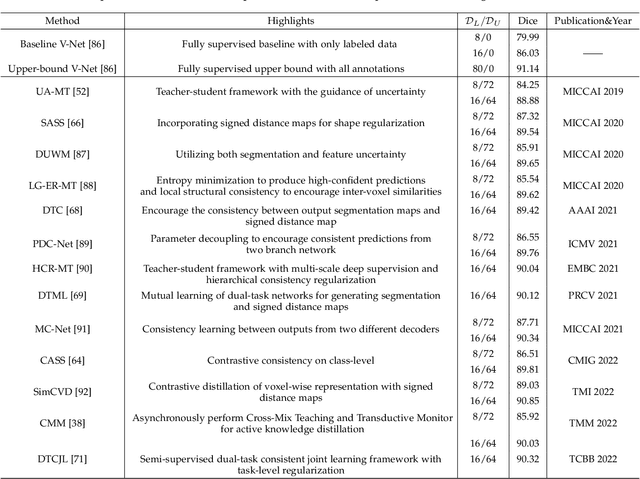
Uncertainty-Guided Mutual Consistency Learning for Semi-Supervised Medical Image Segmentation
Dec 05, 2021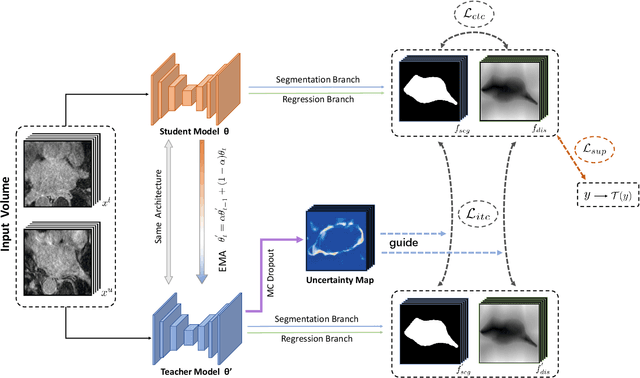
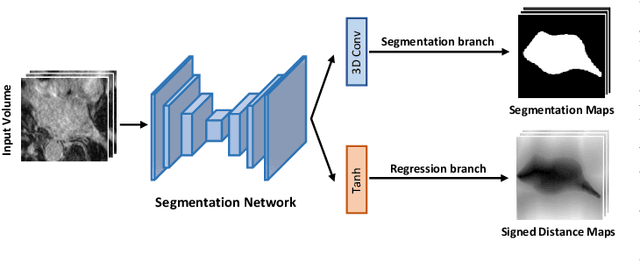
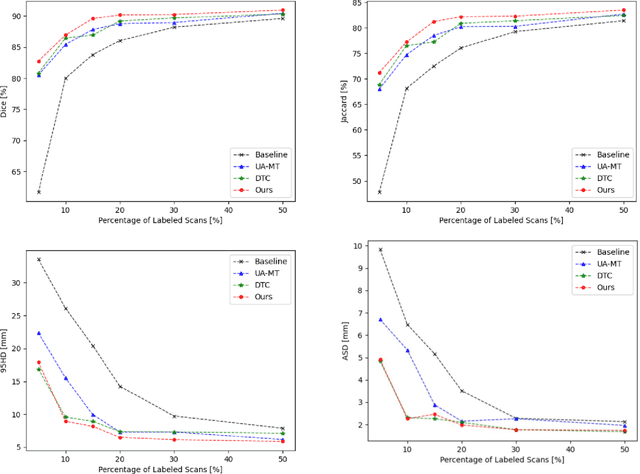
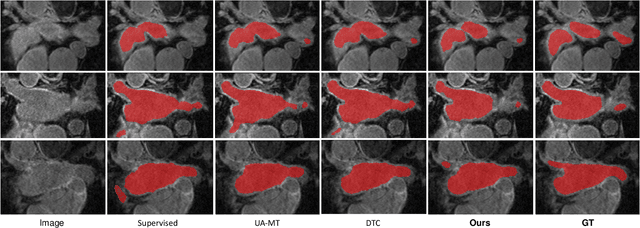
Abstract:Medical image segmentation is a fundamental and critical step in many clinical approaches. Semi-supervised learning has been widely applied to medical image segmentation tasks since it alleviates the heavy burden of acquiring expert-examined annotations and takes the advantage of unlabeled data which is much easier to acquire. Although consistency learning has been proven to be an effective approach by enforcing an invariance of predictions under different distributions, existing approaches cannot make full use of region-level shape constraint and boundary-level distance information from unlabeled data. In this paper, we propose a novel uncertainty-guided mutual consistency learning framework to effectively exploit unlabeled data by integrating intra-task consistency learning from up-to-date predictions for self-ensembling and cross-task consistency learning from task-level regularization to exploit geometric shape information. The framework is guided by the estimated segmentation uncertainty of models to select out relatively certain predictions for consistency learning, so as to effectively exploit more reliable information from unlabeled data. We extensively validate our proposed method on two publicly available benchmark datasets: Left Atrium Segmentation (LA) dataset and Brain Tumor Segmentation (BraTS) dataset. Experimental results demonstrate that our method achieves performance gains by leveraging unlabeled data and outperforms existing semi-supervised segmentation methods.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge