Rongxiao Zhang
Robust Real-time Segmentation of Bio-Morphological Features in Human Cherenkov Imaging during Radiotherapy via Deep Learning
Sep 09, 2024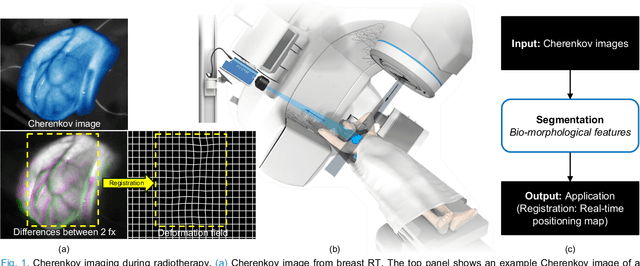
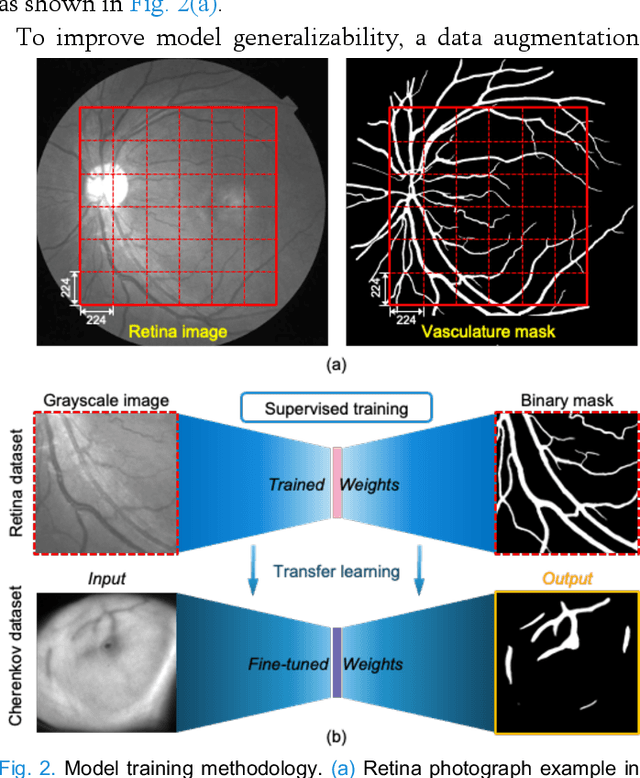

Abstract:Cherenkov imaging enables real-time visualization of megavoltage X-ray or electron beam delivery to the patient during Radiation Therapy (RT). Bio-morphological features, such as vasculature, seen in these images are patient-specific signatures that can be used for verification of positioning and motion management that are essential to precise RT treatment. However until now, no concerted analysis of this biological feature-based tracking was utilized because of the slow speed and accuracy of conventional image processing for feature segmentation. This study demonstrated the first deep learning framework for such an application, achieving video frame rate processing. To address the challenge of limited annotation of these features in Cherenkov images, a transfer learning strategy was applied. A fundus photography dataset including 20,529 patch retina images with ground-truth vessel annotation was used to pre-train a ResNet segmentation framework. Subsequently, a small Cherenkov dataset (1,483 images from 212 treatment fractions of 19 breast cancer patients) with known annotated vasculature masks was used to fine-tune the model for accurate segmentation prediction. This deep learning framework achieved consistent and rapid segmentation of Cherenkov-imaged bio-morphological features on another 19 patients, including subcutaneous veins, scars, and pigmented skin. Average segmentation by the model achieved Dice score of 0.85 and required less than 0.7 milliseconds processing time per instance. The model demonstrated outstanding consistency against input image variances and speed compared to conventional manual segmentation methods, laying the foundation for online segmentation in real-time monitoring in a prospective setting.
Cherenkov Imaged Bio-morphological Features Verify Patient Positioning with Deformable Tissue Translocation in Breast Radiotherapy
Sep 09, 2024Abstract:Accurate patient positioning is critical for precise radiotherapy dose delivery, as positioning errors can significantly affect treatment outcomes. This study introduces a novel method for tracking loco-regional tissue deformation through Cherenkov image analysis during fractionated breast cancer radiotherapy. The primary goal was to develop and test an algorithm for Cherenkov-based regional position accuracy quantification, specifically for loco-regional deformations, which lack ideal quantification methods in radiotherapy. Blood vessel detection and segmentation were developed in Cherenkov images using a tissue phantom with incremental movements, and later applied to images from fractionated whole breast radiotherapy in human patients (n=10). A combined rigid and non-rigid registration technique was used to detect inter- and intra-fractional positioning variations. This approach quantified positioning variations in two parts: a global shift from rigid registration and a two-dimensional variation map of loco-regional deformation from non-rigid registration. The methodology was validated using an anthropomorphic chest phantom experiment, where known treatment couch translations and respiratory motion were simulated to assess inter- and intra-fractional uncertainties, yielding an average accuracy of 0.83 mm for couch translations up to 20 mm. Analysis of clinical Cherenkov data from ten breast cancer patients showed an inter-fraction setup variation of 3.7 plus minus 2.4 mm relative to the first fraction and loco-regional deformations (95th percentile) of up to 3.3 plus minus 1.9 mm. This study presents a Cherenkov-based approach to quantify global and local positioning variations, demonstrating feasibility in addressing loco-regional deformations that conventional imaging techniques fail to capture.
Cycle-consistent Generative Adversarial Network Synthetic CT for MR-only Adaptive Radiation Therapy on MR-Linac
Dec 03, 2023Abstract:Purpose: This study assesses the effectiveness of Deep Learning (DL) for creating synthetic CT (sCT) images in MR-guided adaptive radiation therapy (MRgART). Methods: A Cycle-GAN model was trained with MRI and CT scan slices from MR-LINAC treatments, generating sCT volumes. The analysis involved retrospective treatment plan data from patients with various tumors. sCT images were compared with standard CT scans using mean absolute error in Hounsfield Units (HU) and image similarity metrics (SSIM, PSNR, NCC). sCT volumes were integrated into a clinical treatment system for dosimetric re-evaluation. Results: The model, trained on 8405 frames from 57 patients and tested on 357 sCT frames from 17 patients, showed sCTs comparable to dCTs in electron density and structural similarity with MRI scans. The MAE between sCT and dCT was 49.2 +/- 13.2 HU, with sCT NCC exceeding dCT by 0.06, and SSIM and PSNR at 0.97 +/- 0.01 and 19.9 +/- 1.6 respectively. Dosimetric evaluations indicated minimal differences between sCTs and dCTs, with sCTs showing better air-bubble reconstruction. Conclusions: DL-based sCT generation on MR-Linacs is accurate for dose calculation and optimization in MRgART. This could facilitate MR-only treatment planning, enhancing simulation and adaptive planning efficiency on MR-Linacs.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge