Brady Hunt
Cycle-consistent Generative Adversarial Network Synthetic CT for MR-only Adaptive Radiation Therapy on MR-Linac
Dec 03, 2023Abstract:Purpose: This study assesses the effectiveness of Deep Learning (DL) for creating synthetic CT (sCT) images in MR-guided adaptive radiation therapy (MRgART). Methods: A Cycle-GAN model was trained with MRI and CT scan slices from MR-LINAC treatments, generating sCT volumes. The analysis involved retrospective treatment plan data from patients with various tumors. sCT images were compared with standard CT scans using mean absolute error in Hounsfield Units (HU) and image similarity metrics (SSIM, PSNR, NCC). sCT volumes were integrated into a clinical treatment system for dosimetric re-evaluation. Results: The model, trained on 8405 frames from 57 patients and tested on 357 sCT frames from 17 patients, showed sCTs comparable to dCTs in electron density and structural similarity with MRI scans. The MAE between sCT and dCT was 49.2 +/- 13.2 HU, with sCT NCC exceeding dCT by 0.06, and SSIM and PSNR at 0.97 +/- 0.01 and 19.9 +/- 1.6 respectively. Dosimetric evaluations indicated minimal differences between sCTs and dCTs, with sCTs showing better air-bubble reconstruction. Conclusions: DL-based sCT generation on MR-Linacs is accurate for dose calculation and optimization in MRgART. This could facilitate MR-only treatment planning, enhancing simulation and adaptive planning efficiency on MR-Linacs.
Fluorescence molecular optomic signatures improve identification of tumors in head and neck specimens
Aug 29, 2022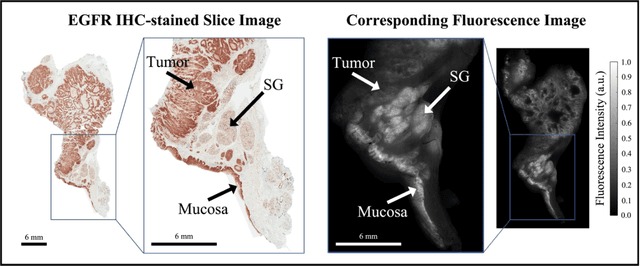
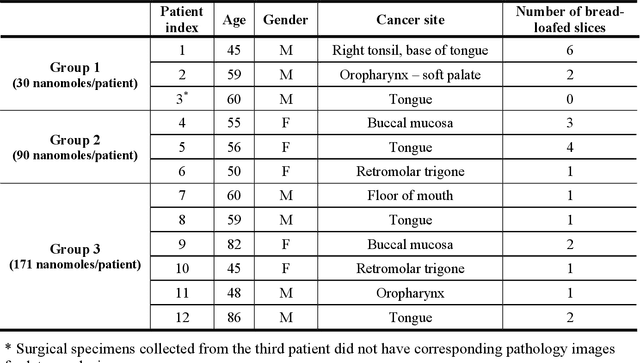
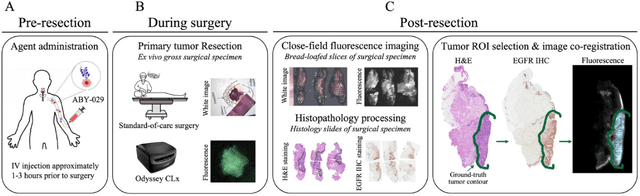
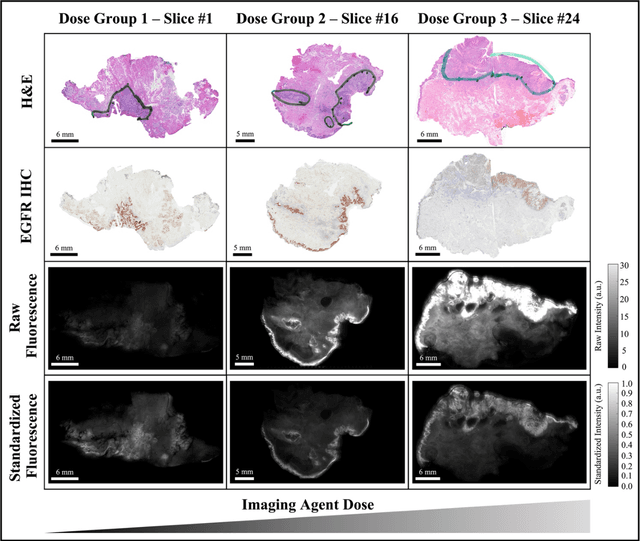
Abstract:In this study, a radiomics approach was extended to optical fluorescence molecular imaging data for tissue classification, termed 'optomics'. Fluorescence molecular imaging is emerging for precise surgical guidance during head and neck squamous cell carcinoma (HNSCC) resection. However, the tumor-to-normal tissue contrast is confounded by intrinsic physiological limitations of heterogeneous expression of the target molecule, epidermal growth factor receptor (EGFR). Optomics seek to improve tumor identification by probing textural pattern differences in EGFR expression conveyed by fluorescence. A total of 1,472 standardized optomic features were extracted from fluorescence image samples. A supervised machine learning pipeline involving a support vector machine classifier was trained with 25 top-ranked features selected by minimum redundancy maximum relevance criterion. Model predictive performance was compared to fluorescence intensity thresholding method by classifying testing set image patches of resected tissue with histologically confirmed malignancy status. The optomics approach provided consistent improvement in prediction accuracy on all test set samples, irrespective of dose, compared to fluorescence intensity thresholding method (mean accuracies of 89% vs. 81%; P = 0.0072). The improved performance demonstrates that extending the radiomics approach to fluorescence molecular imaging data offers a promising image analysis technique for cancer detection in fluorescence-guided surgery.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge