Rodica Pop-Busui
Deep Reinforcement Learning for Closed-Loop Blood Glucose Control
Sep 18, 2020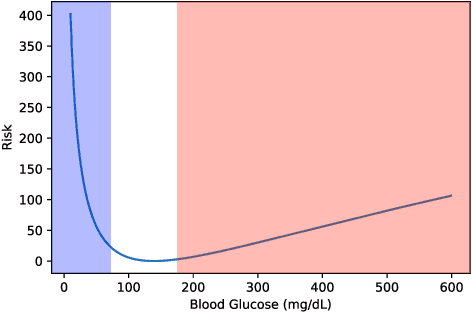
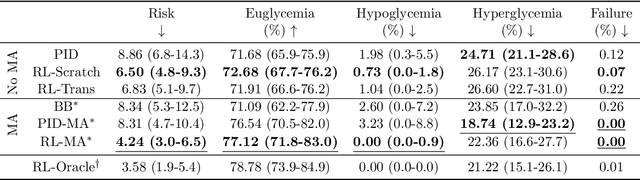
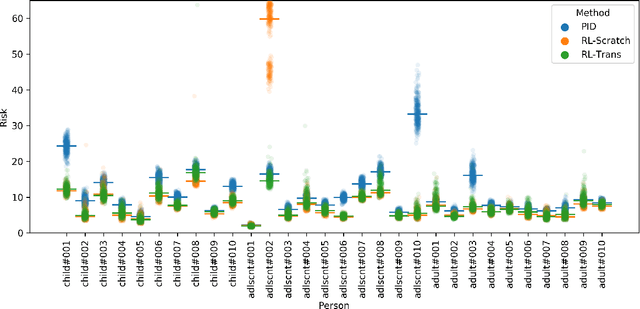
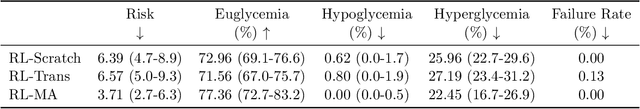
Abstract:People with type 1 diabetes (T1D) lack the ability to produce the insulin their bodies need. As a result, they must continually make decisions about how much insulin to self-administer to adequately control their blood glucose levels. Longitudinal data streams captured from wearables, like continuous glucose monitors, can help these individuals manage their health, but currently the majority of the decision burden remains on the user. To relieve this burden, researchers are working on closed-loop solutions that combine a continuous glucose monitor and an insulin pump with a control algorithm in an `artificial pancreas.' Such systems aim to estimate and deliver the appropriate amount of insulin. Here, we develop reinforcement learning (RL) techniques for automated blood glucose control. Through a series of experiments, we compare the performance of different deep RL approaches to non-RL approaches. We highlight the flexibility of RL approaches, demonstrating how they can adapt to new individuals with little additional data. On over 2.1 million hours of data from 30 simulated patients, our RL approach outperforms baseline control algorithms: leading to a decrease in median glycemic risk of nearly 50% from 8.34 to 4.24 and a decrease in total time hypoglycemic of 99.8%, from 4,610 days to 6. Moreover, these approaches are able to adapt to predictable meal times (decreasing average risk by an additional 24% as meals increase in predictability). This work demonstrates the potential of deep RL to help people with T1D manage their blood glucose levels without requiring expert knowledge. All of our code is publicly available, allowing for replication and extension.
Deep Multi-Output Forecasting: Learning to Accurately Predict Blood Glucose Trajectories
Jun 14, 2018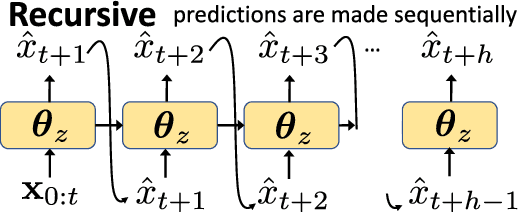
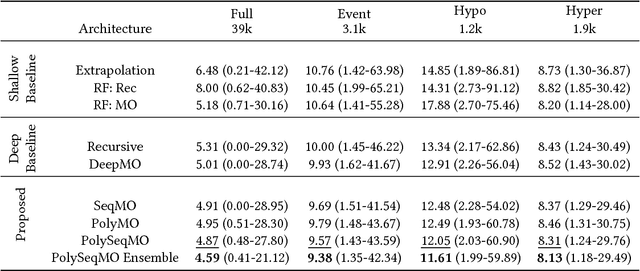
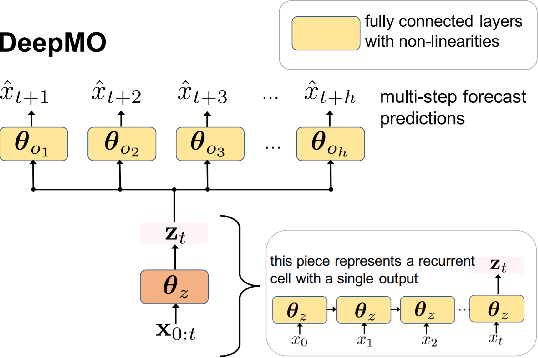
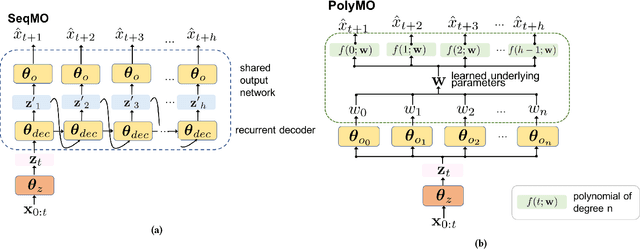
Abstract:In many forecasting applications, it is valuable to predict not only the value of a signal at a certain time point in the future, but also the values leading up to that point. This is especially true in clinical applications, where the future state of the patient can be less important than the patient's overall trajectory. This requires multi-step forecasting, a forecasting variant where one aims to predict multiple values in the future simultaneously. Standard methods to accomplish this can propagate error from prediction to prediction, reducing quality over the long term. In light of these challenges, we propose multi-output deep architectures for multi-step forecasting in which we explicitly model the distribution of future values of the signal over a prediction horizon. We apply these techniques to the challenging and clinically relevant task of blood glucose forecasting. Through a series of experiments on a real-world dataset consisting of 550K blood glucose measurements, we demonstrate the effectiveness of our proposed approaches in capturing the underlying signal dynamics. Compared to existing shallow and deep methods, we find that our proposed approaches improve performance individually and capture complementary information, leading to a large improvement over the baseline when combined (4.87 vs. 5.31 absolute percentage error (APE)). Overall, the results suggest the efficacy of our proposed approach in predicting blood glucose level and multi-step forecasting more generally.
Contextual Motifs: Increasing the Utility of Motifs using Contextual Data
Jul 31, 2017
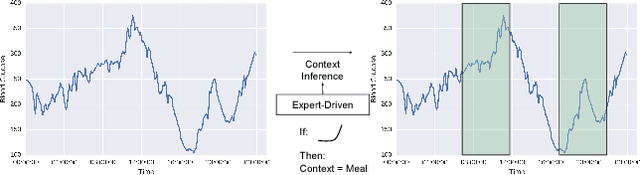
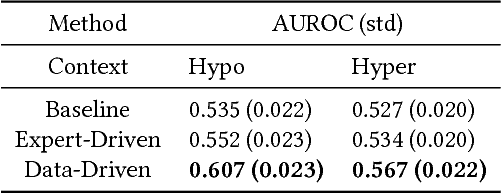
Abstract:Motifs are a powerful tool for analyzing physiological waveform data. Standard motif methods, however, ignore important contextual information (e.g., what the patient was doing at the time the data were collected). We hypothesize that these additional contextual data could increase the utility of motifs. Thus, we propose an extension to motifs, contextual motifs, that incorporates context. Recognizing that, oftentimes, context may be unobserved or unavailable, we focus on methods to jointly infer motifs and context. Applied to both simulated and real physiological data, our proposed approach improves upon existing motif methods in terms of the discriminative utility of the discovered motifs. In particular, we discovered contextual motifs in continuous glucose monitor (CGM) data collected from patients with type 1 diabetes. Compared to their contextless counterparts, these contextual motifs led to better predictions of hypo- and hyperglycemic events. Our results suggest that even when inferred, context is useful in both a long- and short-term prediction horizon when processing and interpreting physiological waveform data.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge