Richard Palyo
TAI-GAN: A Temporally and Anatomically Informed Generative Adversarial Network for early-to-late frame conversion in dynamic cardiac PET inter-frame motion correction
Feb 14, 2024
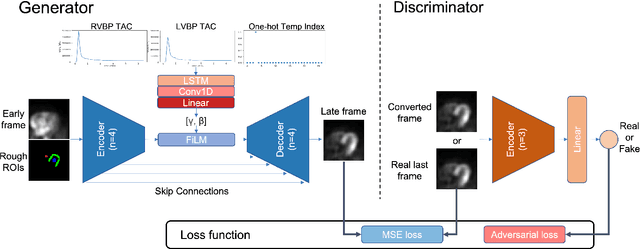
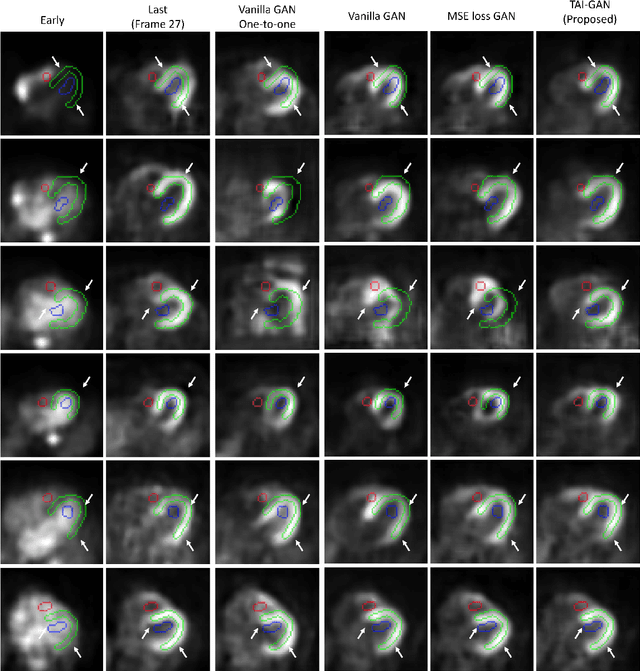
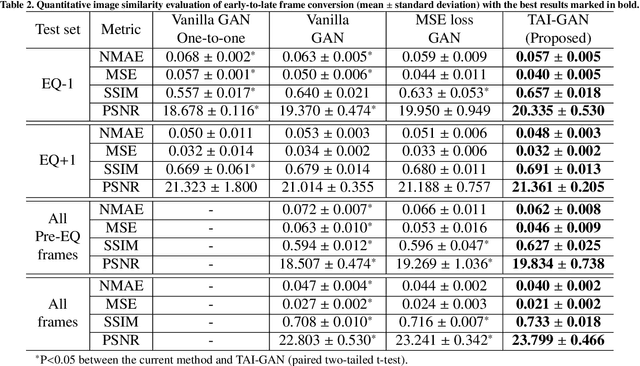
Abstract:Inter-frame motion in dynamic cardiac positron emission tomography (PET) using rubidium-82 (82-Rb) myocardial perfusion imaging impacts myocardial blood flow (MBF) quantification and the diagnosis accuracy of coronary artery diseases. However, the high cross-frame distribution variation due to rapid tracer kinetics poses a considerable challenge for inter-frame motion correction, especially for early frames where intensity-based image registration techniques often fail. To address this issue, we propose a novel method called Temporally and Anatomically Informed Generative Adversarial Network (TAI-GAN) that utilizes an all-to-one mapping to convert early frames into those with tracer distribution similar to the last reference frame. The TAI-GAN consists of a feature-wise linear modulation layer that encodes channel-wise parameters generated from temporal information and rough cardiac segmentation masks with local shifts that serve as anatomical information. Our proposed method was evaluated on a clinical 82-Rb PET dataset, and the results show that our TAI-GAN can produce converted early frames with high image quality, comparable to the real reference frames. After TAI-GAN conversion, the motion estimation accuracy and subsequent myocardial blood flow (MBF) quantification with both conventional and deep learning-based motion correction methods were improved compared to using the original frames.
TAI-GAN: Temporally and Anatomically Informed GAN for early-to-late frame conversion in dynamic cardiac PET motion correction
Aug 23, 2023Abstract:The rapid tracer kinetics of rubidium-82 ($^{82}$Rb) and high variation of cross-frame distribution in dynamic cardiac positron emission tomography (PET) raise significant challenges for inter-frame motion correction, particularly for the early frames where conventional intensity-based image registration techniques are not applicable. Alternatively, a promising approach utilizes generative methods to handle the tracer distribution changes to assist existing registration methods. To improve frame-wise registration and parametric quantification, we propose a Temporally and Anatomically Informed Generative Adversarial Network (TAI-GAN) to transform the early frames into the late reference frame using an all-to-one mapping. Specifically, a feature-wise linear modulation layer encodes channel-wise parameters generated from temporal tracer kinetics information, and rough cardiac segmentations with local shifts serve as the anatomical information. We validated our proposed method on a clinical $^{82}$Rb PET dataset and found that our TAI-GAN can produce converted early frames with high image quality, comparable to the real reference frames. After TAI-GAN conversion, motion estimation accuracy and clinical myocardial blood flow (MBF) quantification were improved compared to using the original frames. Our code is published at https://github.com/gxq1998/TAI-GAN.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge