Rahmetullah Varol
An ensemble deep learning approach to detect tumors on Mohs micrographic surgery slides
Apr 07, 2025Abstract:Mohs micrographic surgery (MMS) is the gold standard technique for removing high risk nonmelanoma skin cancer however, intraoperative histopathological examination demands significant time, effort, and professionality. The objective of this study is to develop a deep learning model to detect basal cell carcinoma (BCC) and artifacts on Mohs slides. A total of 731 Mohs slides from 51 patients with BCCs were used in this study, with 91 containing tumor and 640 without tumor which was defined as non-tumor. The dataset was employed to train U-Net based models that segment tumor and non-tumor regions on the slides. The segmented patches were classified as tumor, or non-tumor to produce predictions for whole slide images (WSIs). For the segmentation phase, the deep learning model success was measured using a Dice score with 0.70 and 0.67 value, area under the curve (AUC) score with 0.98 and 0.96 for tumor and non-tumor, respectively. For the tumor classification, an AUC of 0.98 for patch-based detection, and AUC of 0.91 for slide-based detection was obtained on the test dataset. We present an AI system that can detect tumors and non-tumors in Mohs slides with high success. Deep learning can aid Mohs surgeons and dermatopathologists in making more accurate decisions.
Automated Onychomycosis Detection Using Deep Neural Networks
Jul 13, 2021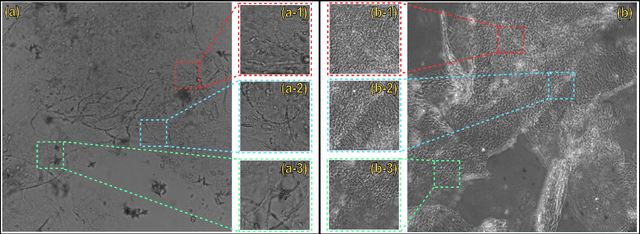
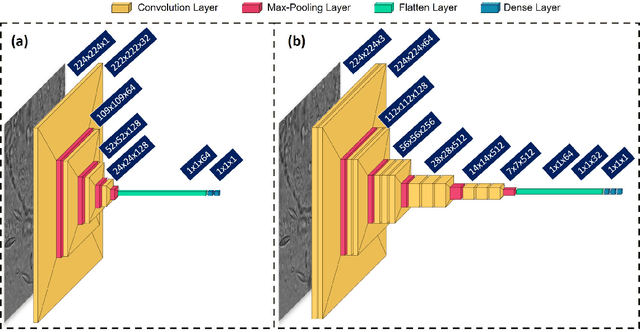
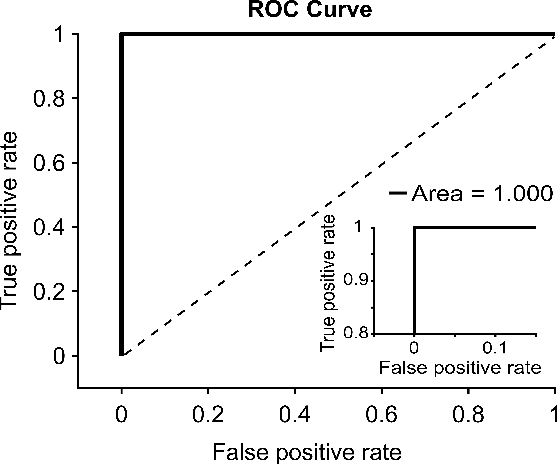

Abstract:Clinical dermatology, still relies heavily on manual introspection of fungi within a Potassium Hydroxide (KOH) solution using a brightfield microscope. However, this method takes a long time, is based on the experience of the clinician, and has a low accuracy. With the increase of neural network applications in the field of clinical microscopy it is now possible to automate such manual processes increasing both efficiency and accuracy. This study presents a deep neural network structure that enables the rapid solutions for these problems and can perform automatic fungi detection in grayscale images without colorants. Microscopic images of 81 fungi and 235 ceratine were collected. Then, smaller patches were extracted containing 2062 fungi and 2142 ceratine. In order to detect fungus and ceratine, two models were created one of which was a custom neural network and the other was based on the VGG16 architecture. The developed custom model had 99.84% accuracy, and an area under the curve (AUC) value of 1.00, while the VGG16 model had 98.89% accuracy and an AUC value of 0.99. However, average accuracy and AUC value of clinicians is 72.8% and 0.87 respectively. This deep learning model allows the development of an automated system that can detect fungi within microscopic images.
Holographic Cell Stiffness Mapping Using Acoustic Stimulation
Feb 15, 2021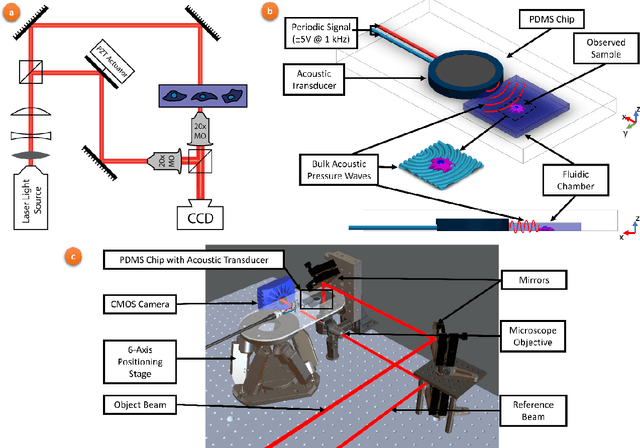
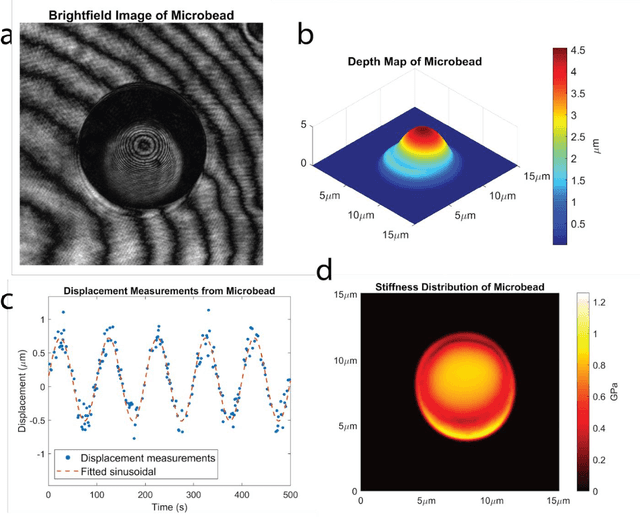
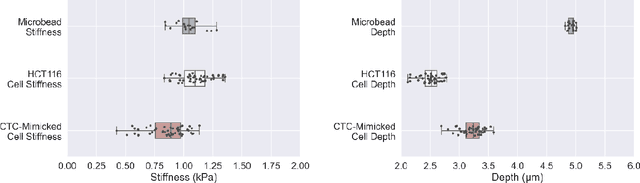
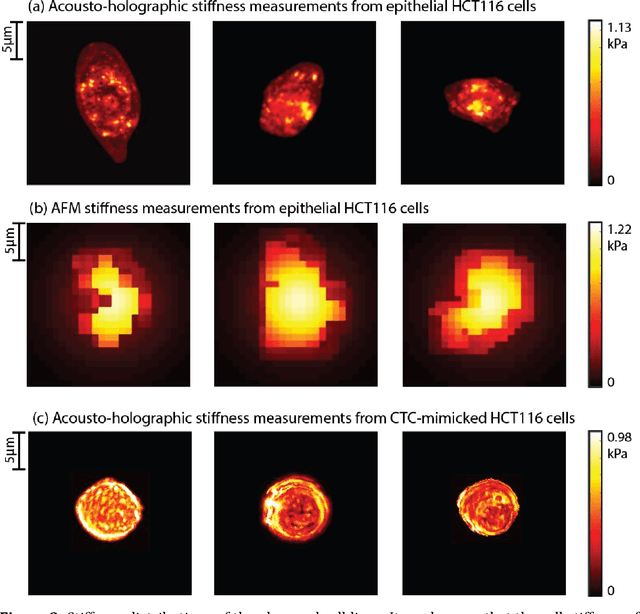
Abstract:Accurate assessment of stiffness distribution is essential due to the critical role of single cell mechanobiology in the regulation of many vital cellular processes such as proliferation, adhesion, migration, and motility. Cell stiffness is one of the fundamental mechanical properties of the cell and is greatly affected by the intracellular tensional forces, cytoskeletal prestress, and cytoskeleton structure. Herein, we propose a novel holographic single-cell stiffness measurement technique that can obtain the stiffness distribution over a cell membrane at high resolution and in real-time. The proposed imaging method coupled with acoustic signals allows us to assess the cell stiffness distribution with a low error margin and label-free manner. We demonstrate the proposed technique on HCT116 (Human Colorectal Carcinoma) cells and CTC-mimicked HCT116 cells by induction with transforming growth factor-beta (TGF-\b{eta}). Validation studies of the proposed approach were carried out on certified polystyrene microbeads with known stiffness levels. Its performance was evaluated in comparison with the AFM results obtained for the relevant cells. When the experimental results were examined, the proposed methodology shows utmost performance over average cell stiffness values for HCT116, and CTC-mimicked HCT116 cells were found as 1.08 kPa, and 0.88 kPa, respectively. The results confirm that CTC-mimicked HCT116 cells lose their adhesion ability to enter the vascular circulation and metastasize. They also exhibit a softer stiffness profile compared to adherent forms of the cancer cells. Hence, the proposed technique is a significant, reliable, and faster alternative for in-vitro cell stiffness characterization tools. It can be utilized for various applications where single-cell analysis is required, such as disease modeling, drug testing, diagnostics, and many more.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge