Qi Ouyang
Transient learning dynamics drive escape from sharp valleys in Stochastic Gradient Descent
Jan 16, 2026Abstract:Stochastic gradient descent (SGD) is central to deep learning, yet the dynamical origin of its preference for flatter, more generalizable solutions remains unclear. Here, by analyzing SGD learning dynamics, we identify a nonequilibrium mechanism governing solution selection. Numerical experiments reveal a transient exploratory phase in which SGD trajectories repeatedly escape sharp valleys and transition toward flatter regions of the loss landscape. By using a tractable physical model, we show that the SGD noise reshapes the landscape into an effective potential that favors flat solutions. Crucially, we uncover a transient freezing mechanism: as training proceeds, growing energy barriers suppress inter-valley transitions and ultimately trap the dynamics within a single basin. Increasing the SGD noise strength delays this freezing, which enhances convergence to flatter minima. Together, these results provide a unified physical framework linking learning dynamics, loss-landscape geometry, and generalization, and suggest principles for the design of more effective optimization algorithms.
Unsupervised single-particle deep clustering via statistical manifold learning
Dec 31, 2016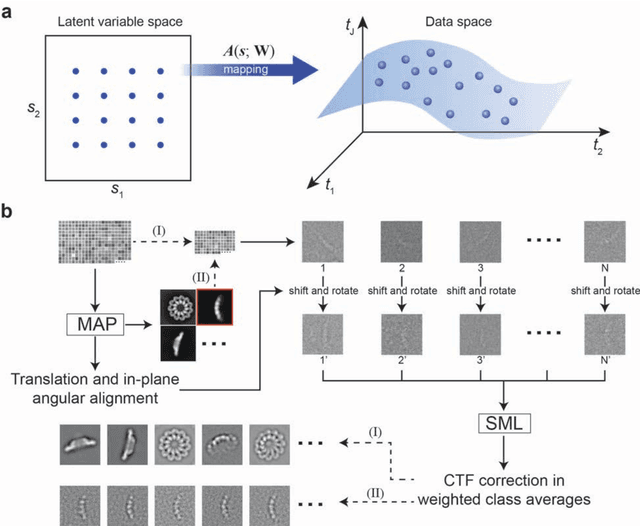
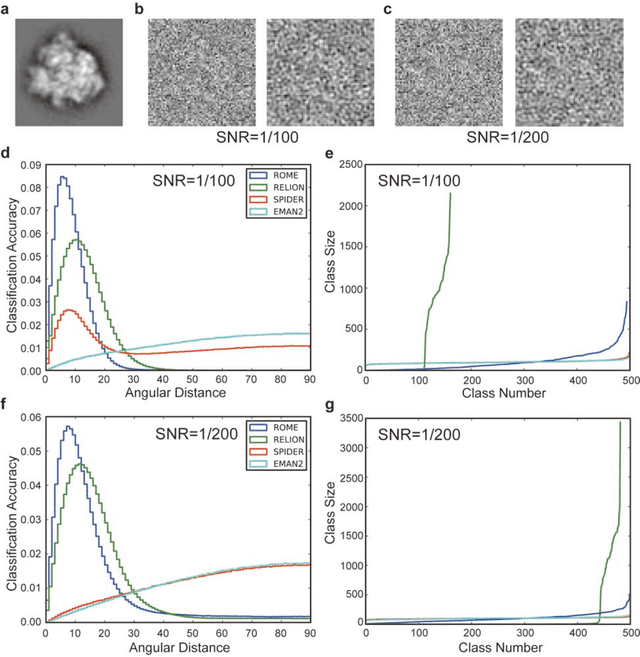
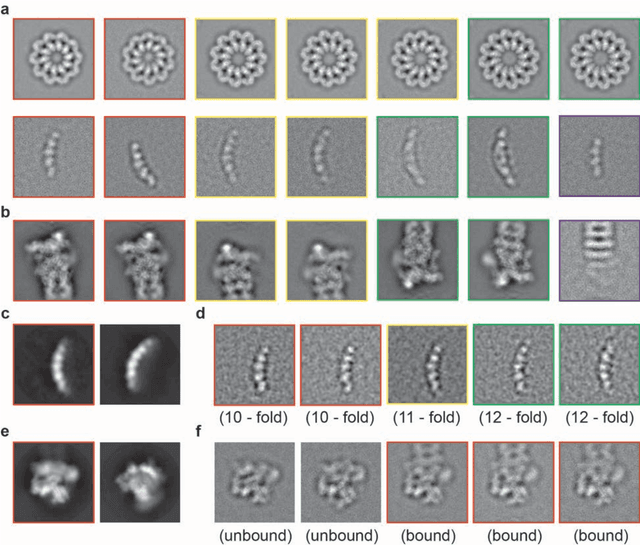
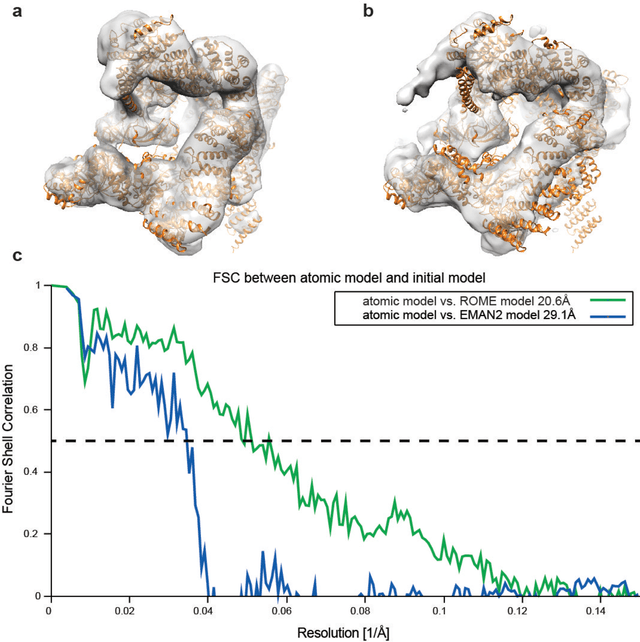
Abstract:Motivation: Structural heterogeneity in single-particle cryo-electron microscopy (cryo-EM) data represents a major challenge for high-resolution structure determination. Unsupervised classification may serve as the first step in the assessment of structural heterogeneity. Traditional algorithms for unsupervised classification, such as K-means clustering and maximum likelihood optimization, may classify images into wrong classes with decreasing signal-to-noise-ratio (SNR) in the image data, yet demand increased cost in computation. Overcoming these limitations requires further development on clustering algorithms for high-performance cryo-EM data analysis. Results: Here we introduce a statistical manifold learning algorithm for unsupervised single-particle deep clustering. We show that statistical manifold learning improves classification accuracy by about 40% in the absence of input references for lower SNR data. Applications to several experimental datasets suggest that our deep clustering approach can detect subtle structural difference among classes. Through code optimization over the Intel high-performance computing (HPC) processors, our software implementation can generate thousands of reference-free class averages within several hours from hundreds of thousands of single-particle cryo-EM images, which allows significant improvement in ab initio 3D reconstruction resolution and quality. Our approach has been successfully applied in several structural determination projects. We expect that it provides a powerful computational tool in analyzing highly heterogeneous structural data and assisting in computational purification of single-particle datasets for high-resolution reconstruction.
* 29 pages, 5 figures
A deep convolutional neural network approach to single-particle recognition in cryo-electron microscopy
Dec 31, 2016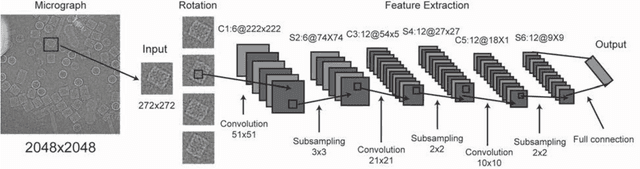
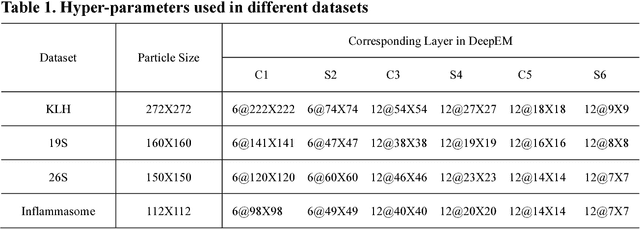
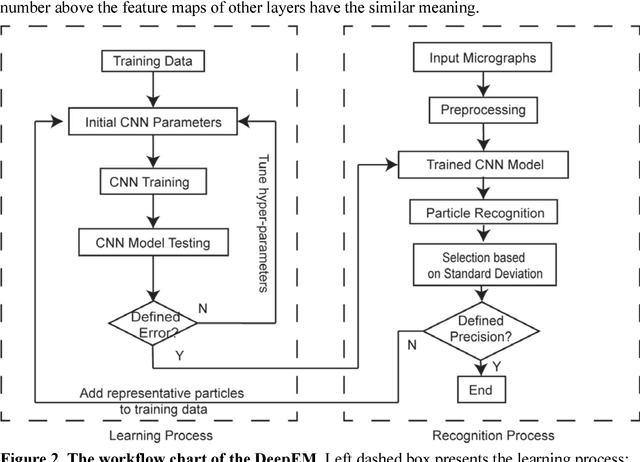
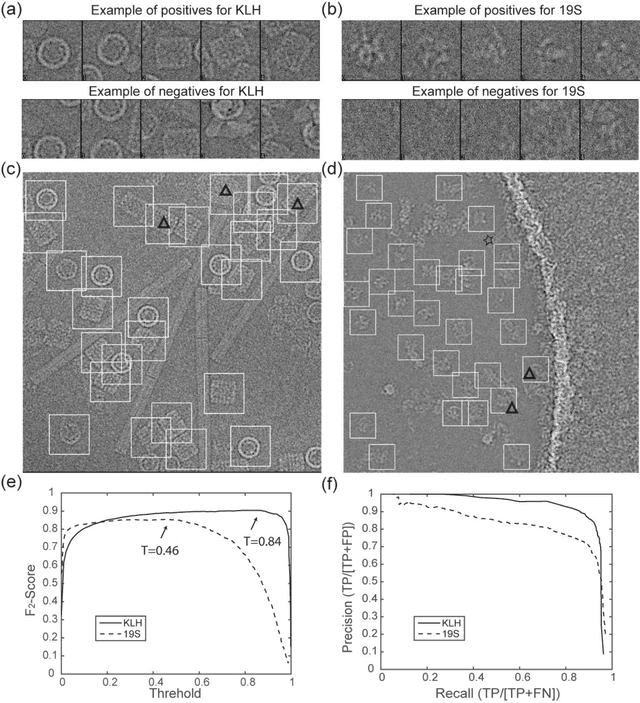
Abstract:Background: Single-particle cryo-electron microscopy (cryo-EM) has become a popular tool for structural determination of biological macromolecular complexes. High-resolution cryo-EM reconstruction often requires hundreds of thousands of single-particle images. Particle extraction from experimental micrographs thus can be laborious and presents a major practical bottleneck in cryo-EM structural determination. Existing computational methods of particle picking often use low-resolution templates as inputs for particle matching, making it possible to cause reference-dependent bias. It is critical to develop a highly efficient template-free method to automatically recognize particle images from cryo-EM micrographs. Results: We developed a deep learning-based algorithmic framework, DeepEM, for single-particle recognition from noisy cryo-EM micrographs, enabling automated particle picking, selection and verification in an integrated fashion. The kernel of DeepEM is built upon a convolutional neural network (CNN) of eight layers, which can be recursively trained to be highly "knowledgeable". Our approach exhibits improved performance and high precision when tested on the standard KLH dataset. Application of DeepEM to several challenging experimental cryo-EM datasets demonstrates its capability in avoiding selection of un-wanted particles and non-particles even when true particles contain fewer features. Conclusions: The DeepEM method derived from a deep CNN allows automated particle extraction from raw cryo-EM micrographs in the absence of templates, which demonstrated improved performance, objectivity and accuracy. Application of this novel approach is expected to free the labor involved in single-particle verification, thus promoting the efficiency of cryo-EM data processing.
* 26 pages, 6 figures, 1 table
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge