Prashant Warier
Can Artificial Intelligence Reliably Report Chest X-Rays?: Radiologist Validation of an Algorithm trained on 1.2 Million X-Rays
Jul 19, 2018


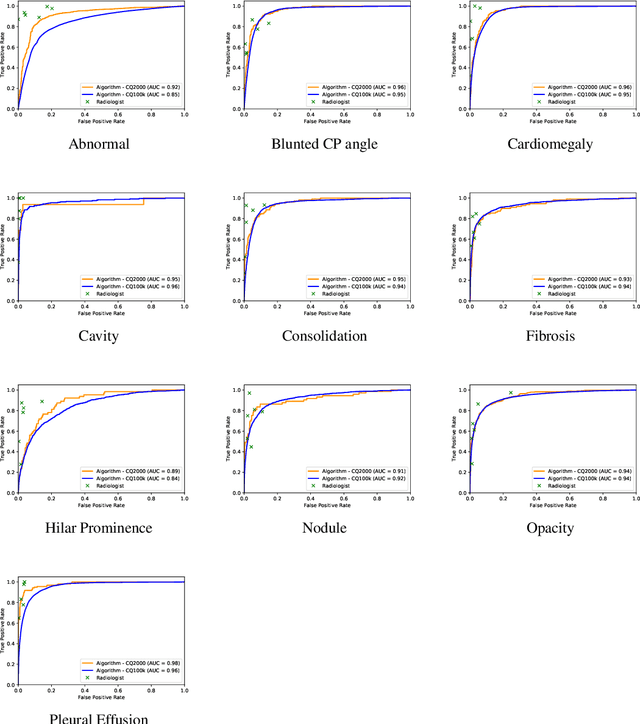
Abstract:Background and Objectives: Chest x-rays are the most commonly performed, cost-effective diagnostic imaging tests ordered by physicians. A clinically validated, automated artificial intelligence system that can reliably separate normal from abnormal would be invaluable in addressing the problem of reporting backlogs and the lack of radiologists in low-resource settings. The aim of this study was to develop and validate a deep learning system to detect chest x-ray abnormalities. Methods: A deep learning system was trained on 1.2 million x-rays and their corresponding radiology reports to identify abnormal x-rays and the following specific abnormalities: blunted costophrenic angle, calcification, cardiomegaly, cavity, consolidation, fibrosis, hilar enlargement, opacity and pleural effusion. The system was tested versus a 3-radiologist majority on an independent, retrospectively collected de-identified set of 2000 x-rays. The primary accuracy measure was area under the ROC curve (AUC), estimated separately for each abnormality as well as for normal versus abnormal reports. Results: The deep learning system demonstrated an AUC of 0.93(CI 0.92-0.94) for detection of abnormal scans, and AUC(CI) of 0.94(0.92-0.97),0.88(0.85-0.91), 0.97(0.95-0.99), 0.92(0.82-1), 0.94(0.91-0.97), 0.92(0.88-0.95), 0.89(0.84-0.94), 0.93(0.92-0.95), 0.98(0.97-1), 0.93(0.0.87-0.99) for the detection of blunted CP angle, calcification, cardiomegaly, cavity, consolidation, fibrosis,hilar enlargement, opacity and pleural effusion respectively. Conclusions and Relevance: Our study shows that a deep learning algorithm trained on a large quantity of labelled data can accurately detect abnormalities on chest x-rays. As these systems further increase in accuracy, the feasibility of using artificial intelligence to extend the reach of chest x-ray interpretation and improve reporting efficiency will increase in tandem.
Development and Validation of Deep Learning Algorithms for Detection of Critical Findings in Head CT Scans
Apr 12, 2018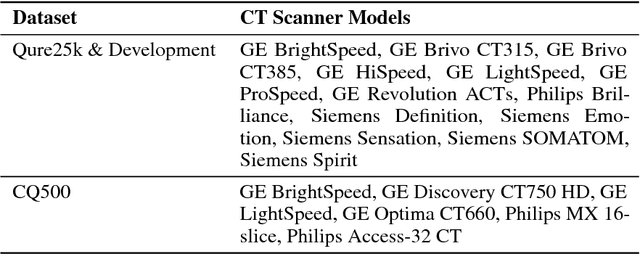
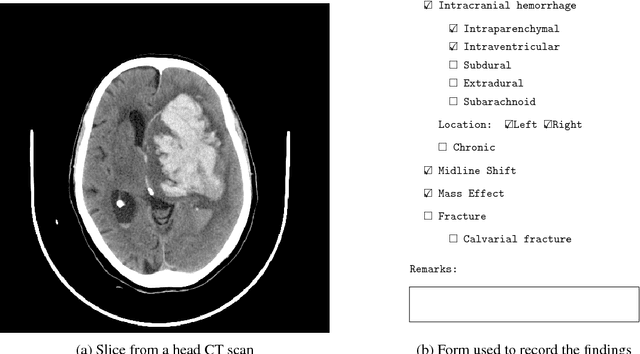
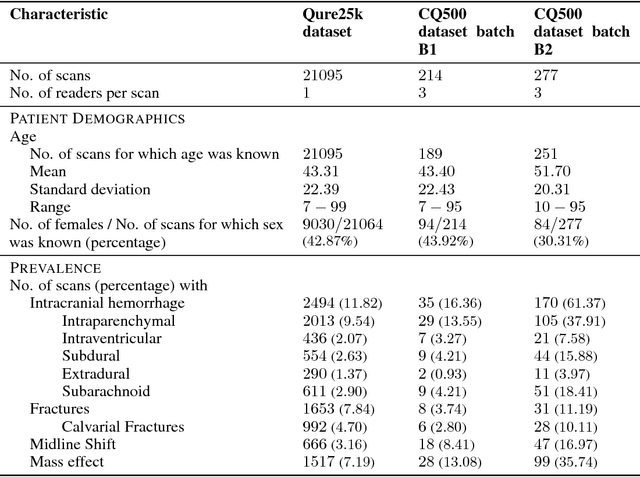
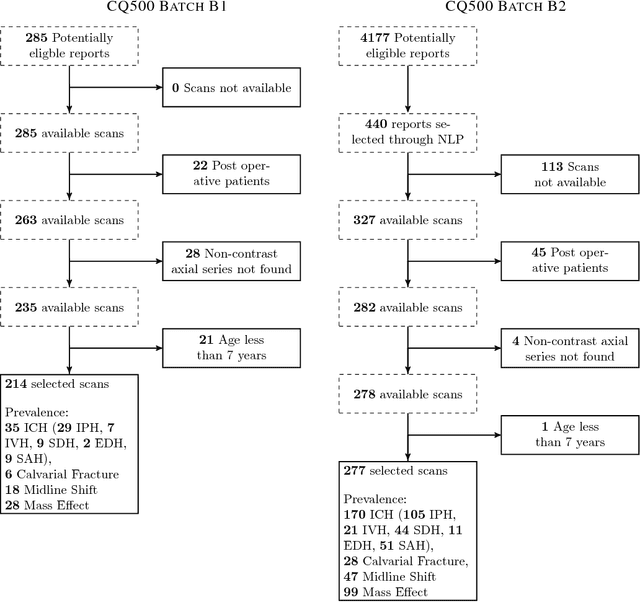
Abstract:Importance: Non-contrast head CT scan is the current standard for initial imaging of patients with head trauma or stroke symptoms. Objective: To develop and validate a set of deep learning algorithms for automated detection of following key findings from non-contrast head CT scans: intracranial hemorrhage (ICH) and its types, intraparenchymal (IPH), intraventricular (IVH), subdural (SDH), extradural (EDH) and subarachnoid (SAH) hemorrhages, calvarial fractures, midline shift and mass effect. Design and Settings: We retrospectively collected a dataset containing 313,318 head CT scans along with their clinical reports from various centers. A part of this dataset (Qure25k dataset) was used to validate and the rest to develop algorithms. Additionally, a dataset (CQ500 dataset) was collected from different centers in two batches B1 & B2 to clinically validate the algorithms. Main Outcomes and Measures: Original clinical radiology report and consensus of three independent radiologists were considered as gold standard for Qure25k and CQ500 datasets respectively. Area under receiver operating characteristics curve (AUC) for each finding was primarily used to evaluate the algorithms. Results: Qure25k dataset contained 21,095 scans (mean age 43.31; 42.87% female) while batches B1 and B2 of CQ500 dataset consisted of 214 (mean age 43.40; 43.92% female) and 277 (mean age 51.70; 30.31% female) scans respectively. On Qure25k dataset, the algorithms achieved AUCs of 0.9194, 0.8977, 0.9559, 0.9161, 0.9288 and 0.9044 for detecting ICH, IPH, IVH, SDH, EDH and SAH respectively. AUCs for the same on CQ500 dataset were 0.9419, 0.9544, 0.9310, 0.9521, 0.9731 and 0.9574 respectively. For detecting calvarial fractures, midline shift and mass effect, AUCs on Qure25k dataset were 0.9244, 0.9276 and 0.8583 respectively, while AUCs on CQ500 dataset were 0.9624, 0.9697 and 0.9216 respectively.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge