Preetham Putha
Comparative Evaluation of Digital and Analog Chest Radiographs to Identify Tuberculosis using Deep Learning Model
Jul 27, 2023
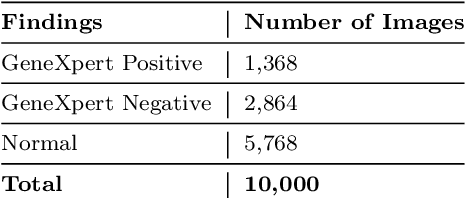
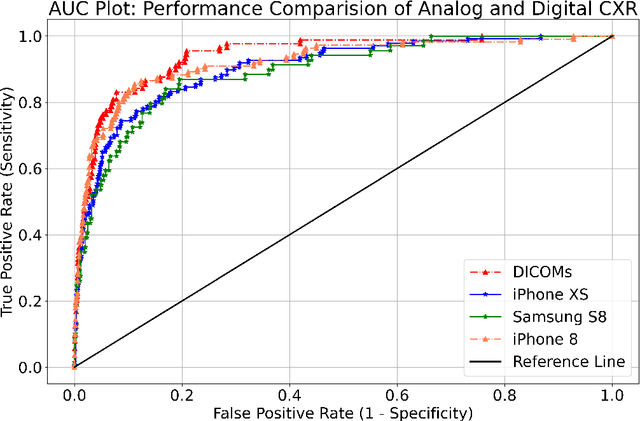
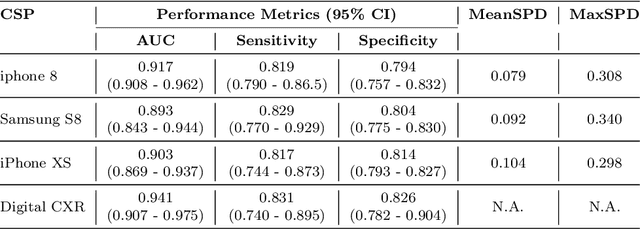
Abstract:Purpose:Chest X-ray (CXR) is an essential tool and one of the most prescribed imaging to detect pulmonary abnormalities, with a yearly estimate of over 2 billion imaging performed worldwide. However, the accurate and timely diagnosis of TB remains an unmet goal. The prevalence of TB is highest in low-middle-income countries, and the requirement of a portable, automated, and reliable solution is required. In this study, we compared the performance of DL-based devices on digital and analog CXR. The evaluated DL-based device can be used in resource-constraint settings. Methods: A total of 10,000 CXR DICOMs(.dcm) and printed photos of the films acquired with three different cellular phones - Samsung S8, iPhone 8, and iPhone XS along with their radiological report were retrospectively collected from various sites across India from April 2020 to March 2021. Results: 10,000 chest X-rays were utilized to evaluate the DL-based device in identifying radiological signs of TB. The AUC of qXR for detecting signs of tuberculosis on the original DICOMs dataset was 0.928 with a sensitivity of 0.841 at a specificity of 0.806. At an optimal threshold, the difference in the AUC of three cellular smartphones with the original DICOMs is 0.024 (2.55%), 0.048 (5.10%), and 0.038 (1.91%). The minimum difference demonstrates the robustness of the DL-based device in identifying radiological signs of TB in both digital and analog CXR.
Can Artificial Intelligence Reliably Report Chest X-Rays?: Radiologist Validation of an Algorithm trained on 1.2 Million X-Rays
Jul 19, 2018


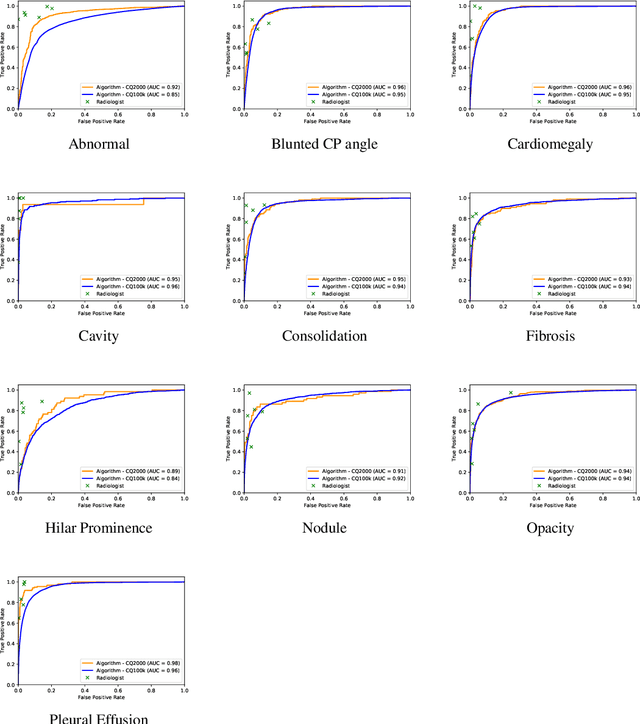
Abstract:Background and Objectives: Chest x-rays are the most commonly performed, cost-effective diagnostic imaging tests ordered by physicians. A clinically validated, automated artificial intelligence system that can reliably separate normal from abnormal would be invaluable in addressing the problem of reporting backlogs and the lack of radiologists in low-resource settings. The aim of this study was to develop and validate a deep learning system to detect chest x-ray abnormalities. Methods: A deep learning system was trained on 1.2 million x-rays and their corresponding radiology reports to identify abnormal x-rays and the following specific abnormalities: blunted costophrenic angle, calcification, cardiomegaly, cavity, consolidation, fibrosis, hilar enlargement, opacity and pleural effusion. The system was tested versus a 3-radiologist majority on an independent, retrospectively collected de-identified set of 2000 x-rays. The primary accuracy measure was area under the ROC curve (AUC), estimated separately for each abnormality as well as for normal versus abnormal reports. Results: The deep learning system demonstrated an AUC of 0.93(CI 0.92-0.94) for detection of abnormal scans, and AUC(CI) of 0.94(0.92-0.97),0.88(0.85-0.91), 0.97(0.95-0.99), 0.92(0.82-1), 0.94(0.91-0.97), 0.92(0.88-0.95), 0.89(0.84-0.94), 0.93(0.92-0.95), 0.98(0.97-1), 0.93(0.0.87-0.99) for the detection of blunted CP angle, calcification, cardiomegaly, cavity, consolidation, fibrosis,hilar enlargement, opacity and pleural effusion respectively. Conclusions and Relevance: Our study shows that a deep learning algorithm trained on a large quantity of labelled data can accurately detect abnormalities on chest x-rays. As these systems further increase in accuracy, the feasibility of using artificial intelligence to extend the reach of chest x-ray interpretation and improve reporting efficiency will increase in tandem.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge