Paulo de Carvalho
A New Approach for Interpretability and Reliability in Clinical Risk Prediction: Acute Coronary Syndrome Scenario
Oct 15, 2021

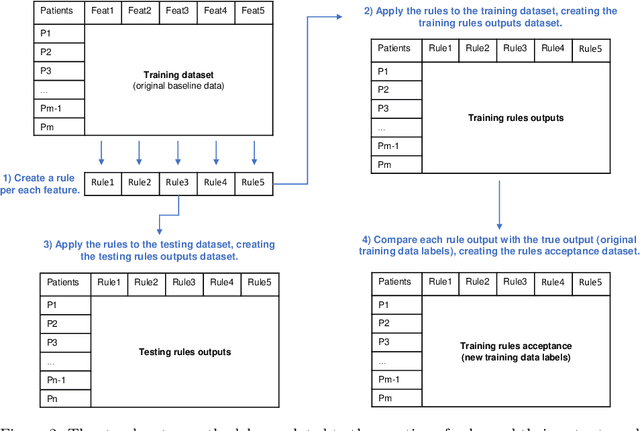
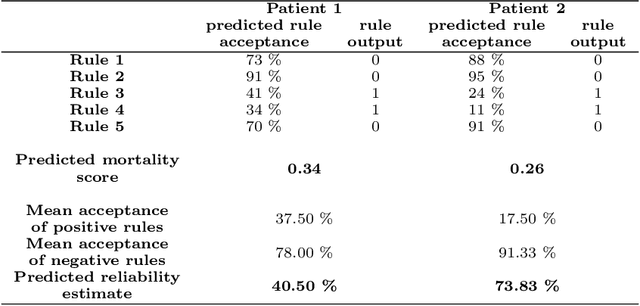
Abstract:We intend to create a new risk assessment methodology that combines the best characteristics of both risk score and machine learning models. More specifically, we aim to develop a method that, besides having a good performance, offers a personalized model and outcome for each patient, presents high interpretability, and incorporates an estimation of the prediction reliability which is not usually available. By combining these features in the same approach we expect that it can boost the confidence of physicians to use such a tool in their daily activity. In order to achieve the mentioned goals, a three-step methodology was developed: several rules were created by dichotomizing risk factors; such rules were trained with a machine learning classifier to predict the acceptance degree of each rule (the probability that the rule is correct) for each patient; that information was combined and used to compute the risk of mortality and the reliability of such prediction. The methodology was applied to a dataset of patients admitted with any type of acute coronary syndromes (ACS), to assess the 30-days all-cause mortality risk. The performance was compared with state-of-the-art approaches: logistic regression (LR), artificial neural network (ANN), and clinical risk score model (Global Registry of Acute Coronary Events - GRACE). The proposed approach achieved testing results identical to the standard LR, but offers superior interpretability and personalization; it also significantly outperforms the GRACE risk model and the standard ANN model. The calibration curve also suggests a very good generalization ability of the obtained model as it approaches the ideal curve. Finally, the reliability estimation of individual predictions presented a great correlation with the misclassifications rate. Those properties may have a beneficial application in other clinical scenarios as well. [abridged]
* Accepted for publication in the Artificial Intelligence in Medicine journal. Abstract abridged to respect the arXiv's characters limit
Classification of Electrical Impedance Tomography Data Using Machine Learning
Aug 02, 2021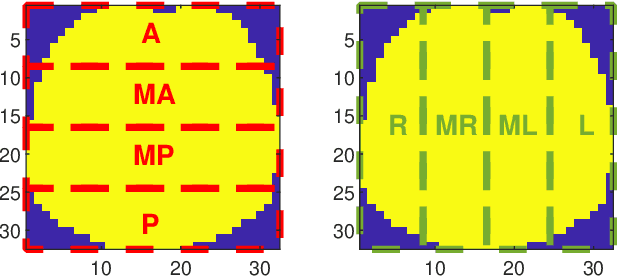
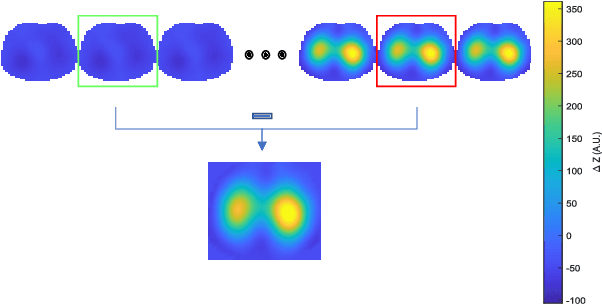
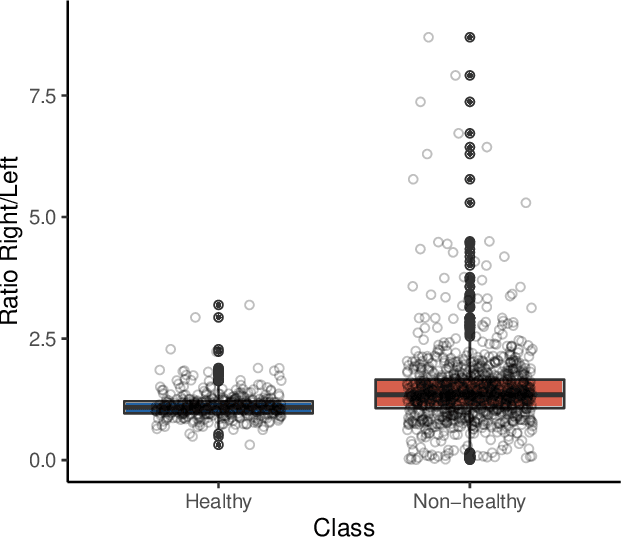
Abstract:Patients suffering from pulmonary diseases typically exhibit pathological lung ventilation in terms of homogeneity. Electrical Impedance Tomography (EIT) is a non-invasive imaging method that allows to analyze and quantify the distribution of ventilation in the lungs. In this article, we present a new approach to promote the use of EIT data and the implementation of new clinical applications for differential diagnosis, with the development of several machine learning models to discriminate between EIT data from healthy and non-healthy subjects. EIT data from 16 subjects were acquired: 5 healthy and 11 non-healthy subjects (with multiple pulmonary conditions). Preliminary results have shown accuracy percentages of 66\% in challenging evaluation scenarios. The results suggest that the pairing of EIT feature engineering methods with machine learning methods could be further explored and applied in the diagnostic and monitoring of patients suffering from lung diseases. Also, we introduce the use of a new feature in the context of EIT data analysis (Impedance Curve Correlation).
Detection of squawks in respiratory sounds of mechanically ventilated COVID-19 patients
Jul 28, 2021



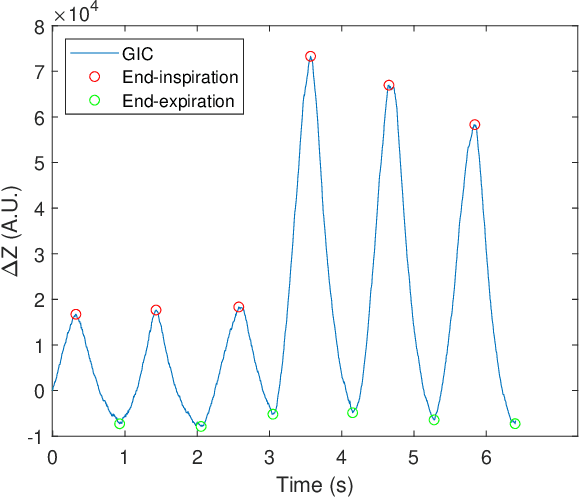
Abstract:Mechanically ventilated patients typically exhibit abnormal respiratory sounds. Squawks are short inspiratory adventitious sounds that may occur in patients with pneumonia, such as COVID-19 patients. In this work we devised a method for squawk detection in mechanically ventilated patients by developing algorithms for respiratory cycle estimation, squawk candidate identification, feature extraction, and clustering. The best classifier reached an F1 of 0.48 at the sound file level and an F1 of 0.66 at the recording session level. These preliminary results are promising, as they were obtained in noisy environments. This method will give health professionals a new feature to assess the potential deterioration of critically ill patients.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge