Patrick Kelly
Self-Supervised Learning for Organs At Risk and Tumor Segmentation with Uncertainty Quantification
May 04, 2023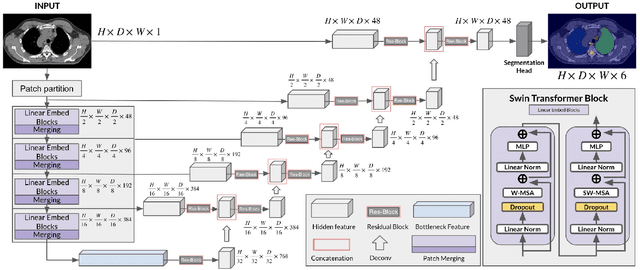
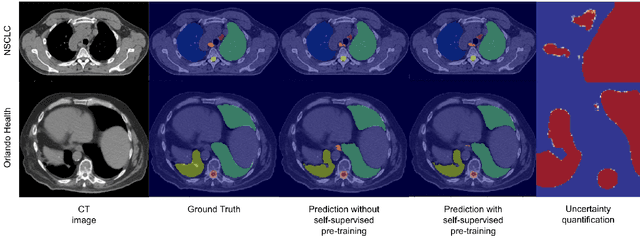
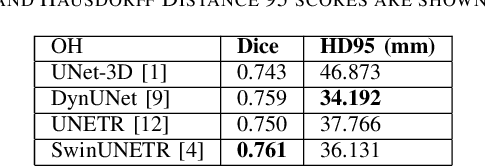
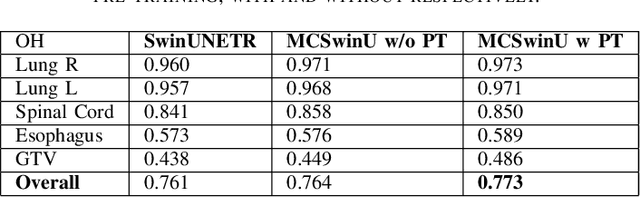
Abstract:In this study, our goal is to show the impact of self-supervised pre-training of transformers for organ at risk (OAR) and tumor segmentation as compared to costly fully-supervised learning. The proposed algorithm is called Monte Carlo Transformer based U-Net (MC-Swin-U). Unlike many other available models, our approach presents uncertainty quantification with Monte Carlo dropout strategy while generating its voxel-wise prediction. We test and validate the proposed model on both public and one private datasets and evaluate the gross tumor volume (GTV) as well as nearby risky organs' boundaries. We show that self-supervised pre-training approach improves the segmentation scores significantly while providing additional benefits for avoiding large-scale annotation costs.
Enhancing Organ at Risk Segmentation with Improved Deep Neural Networks
Feb 03, 2022



Abstract:Organ at risk (OAR) segmentation is a crucial step for treatment planning and outcome determination in radiotherapy treatments of cancer patients. Several deep learning based segmentation algorithms have been developed in recent years, however, U-Net remains the de facto algorithm designed specifically for biomedical image segmentation and has spawned many variants with known weaknesses. In this study, our goal is to present simple architectural changes in U-Net to improve its accuracy and generalization properties. Unlike many other available studies evaluating their algorithms on single center data, we thoroughly evaluate several variations of U-Net as well as our proposed enhanced architecture on multiple data sets for an extensive and reliable study of the OAR segmentation problem. Our enhanced segmentation model includes (a)architectural changes in the loss function, (b)optimization framework, and (c)convolution type. Testing on three publicly available multi-object segmentation data sets, we achieved an average of 80% dice score compared to the baseline U-Net performance of 63%.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge