Nicholas Youngblut
Whole Genome Transformer for Gene Interaction Effects in Microbiome Habitat Specificity
May 09, 2024



Abstract:Leveraging the vast genetic diversity within microbiomes offers unparalleled insights into complex phenotypes, yet the task of accurately predicting and understanding such traits from genomic data remains challenging. We propose a framework taking advantage of existing large models for gene vectorization to predict habitat specificity from entire microbial genome sequences. Based on our model, we develop attribution techniques to elucidate gene interaction effects that drive microbial adaptation to diverse environments. We train and validate our approach on a large dataset of high quality microbiome genomes from different habitats. We not only demonstrate solid predictive performance, but also how sequence-level information of entire genomes allows us to identify gene associations underlying complex phenotypes. Our attribution recovers known important interaction networks and proposes new candidates for experimental follow up.
GeNet: Deep Representations for Metagenomics
Jan 30, 2019
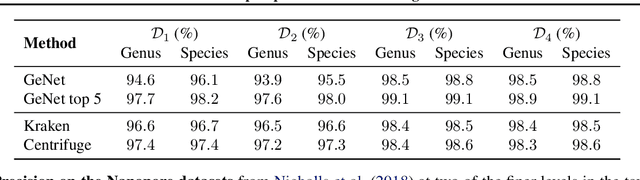
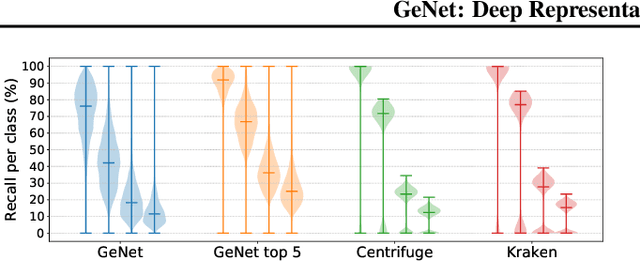
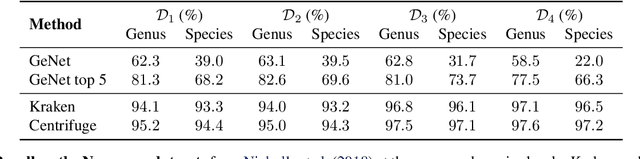
Abstract:We introduce GeNet, a method for shotgun metagenomic classification from raw DNA sequences that exploits the known hierarchical structure between labels for training. We provide a comparison with state-of-the-art methods Kraken and Centrifuge on datasets obtained from several sequencing technologies, in which dataset shift occurs. We show that GeNet obtains competitive precision and good recall, with orders of magnitude less memory requirements. Moreover, we show that a linear model trained on top of representations learned by GeNet achieves recall comparable to state-of-the-art methods on the aforementioned datasets, and achieves over 90% accuracy in a challenging pathogen detection problem. This provides evidence of the usefulness of the representations learned by GeNet for downstream biological tasks.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge