Nguyen Tuan Anh Tran
Clinical Domain Knowledge-Derived Template Improves Post Hoc AI Explanations in Pneumothorax Classification
Mar 26, 2024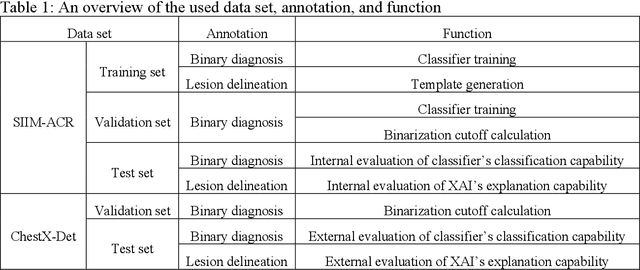

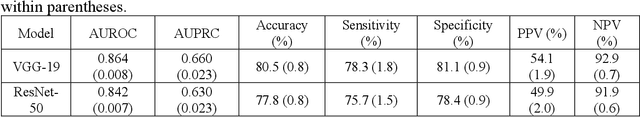

Abstract:Background: Pneumothorax is an acute thoracic disease caused by abnormal air collection between the lungs and chest wall. To address the opaqueness often associated with deep learning (DL) models, explainable artificial intelligence (XAI) methods have been introduced to outline regions related to pneumothorax diagnoses made by DL models. However, these explanations sometimes diverge from actual lesion areas, highlighting the need for further improvement. Method: We propose a template-guided approach to incorporate the clinical knowledge of pneumothorax into model explanations generated by XAI methods, thereby enhancing the quality of these explanations. Utilizing one lesion delineation created by radiologists, our approach first generates a template that represents potential areas of pneumothorax occurrence. This template is then superimposed on model explanations to filter out extraneous explanations that fall outside the template's boundaries. To validate its efficacy, we carried out a comparative analysis of three XAI methods with and without our template guidance when explaining two DL models in two real-world datasets. Results: The proposed approach consistently improved baseline XAI methods across twelve benchmark scenarios built on three XAI methods, two DL models, and two datasets. The average incremental percentages, calculated by the performance improvements over the baseline performance, were 97.8% in Intersection over Union (IoU) and 94.1% in Dice Similarity Coefficient (DSC) when comparing model explanations and ground-truth lesion areas. Conclusions: In the context of pneumothorax diagnoses, we proposed a template-guided approach for improving AI explanations. We anticipate that our template guidance will forge a fresh approach to elucidating AI models by integrating clinical domain expertise.
Leveraging Anatomical Constraints with Uncertainty for Pneumothorax Segmentation
Nov 26, 2023



Abstract:Pneumothorax is a medical emergency caused by abnormal accumulation of air in the pleural space - the potential space between the lungs and chest wall. On 2D chest radiographs, pneumothorax occurs within the thoracic cavity and outside of the mediastinum and we refer to this area as "lung+ space". While deep learning (DL) has increasingly been utilized to segment pneumothorax lesions in chest radiographs, many existing DL models employ an end-to-end approach. These models directly map chest radiographs to clinician-annotated lesion areas, often neglecting the vital domain knowledge that pneumothorax is inherently location-sensitive. We propose a novel approach that incorporates the lung+ space as a constraint during DL model training for pneumothorax segmentation on 2D chest radiographs. To circumvent the need for additional annotations and to prevent potential label leakage on the target task, our method utilizes external datasets and an auxiliary task of lung segmentation. This approach generates a specific constraint of lung+ space for each chest radiograph. Furthermore, we have incorporated a discriminator to eliminate unreliable constraints caused by the domain shift between the auxiliary and target datasets. Our results demonstrated significant improvements, with average performance gains of 4.6%, 3.6%, and 3.3% regarding Intersection over Union (IoU), Dice Similarity Coefficient (DSC), and Hausdorff Distance (HD). Our research underscores the significance of incorporating medical domain knowledge about the location-specific nature of pneumothorax to enhance DL-based lesion segmentation.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge