Neha Koonjoo
Uncertainty Estimation for Deep Learning Image Reconstruction using a Local Lipschitz Metric
May 12, 2023Abstract:The use of deep learning approaches for image reconstruction is of contemporary interest in radiology, especially for approaches that solve inverse problems associated with imaging. In deployment, these models may be exposed to input distributions that are widely shifted from training data, due in part to data biases or drifts. We propose a metric based on local Lipschitz determined from a single trained model that can be used to estimate the model uncertainty for image reconstructions. We demonstrate a monotonic relationship between the local Lipschitz value and Mean Absolute Error and show that this method can be used to provide a threshold that determines whether a given DL reconstruction approach was well suited to the task. Our uncertainty estimation method can be used to identify out-of-distribution test samples, relate information regarding epistemic uncertainties, and guide proper data augmentation. Quantifying uncertainty of learned reconstruction approaches is especially pertinent to the medical domain where reconstructed images must remain diagnostically accurate.
Synthetic Low-Field MRI Super-Resolution Via Nested U-Net Architecture
Nov 28, 2022Abstract:Low-field (LF) MRI scanners have the power to revolutionize medical imaging by providing a portable and cheaper alternative to high-field MRI scanners. However, such scanners are usually significantly noisier and lower quality than their high-field counterparts. The aim of this paper is to improve the SNR and overall image quality of low-field MRI scans to improve diagnostic capability. To address this issue, we propose a Nested U-Net neural network architecture super-resolution algorithm that outperforms previously suggested deep learning methods with an average PSNR of 78.83 and SSIM of 0.9551. We tested our network on artificial noisy downsampled synthetic data from a major T1 weighted MRI image dataset called the T1-mix dataset. One board-certified radiologist scored 25 images on the Likert scale (1-5) assessing overall image quality, anatomical structure, and diagnostic confidence across our architecture and other published works (SR DenseNet, Generator Block, SRCNN, etc.). We also introduce a new type of loss function called natural log mean squared error (NLMSE). In conclusion, we present a more accurate deep learning method for single image super-resolution applied to synthetic low-field MRI via a Nested U-Net architecture.
On Real-time Image Reconstruction with Neural Networks for MRI-guided Radiotherapy
Feb 10, 2022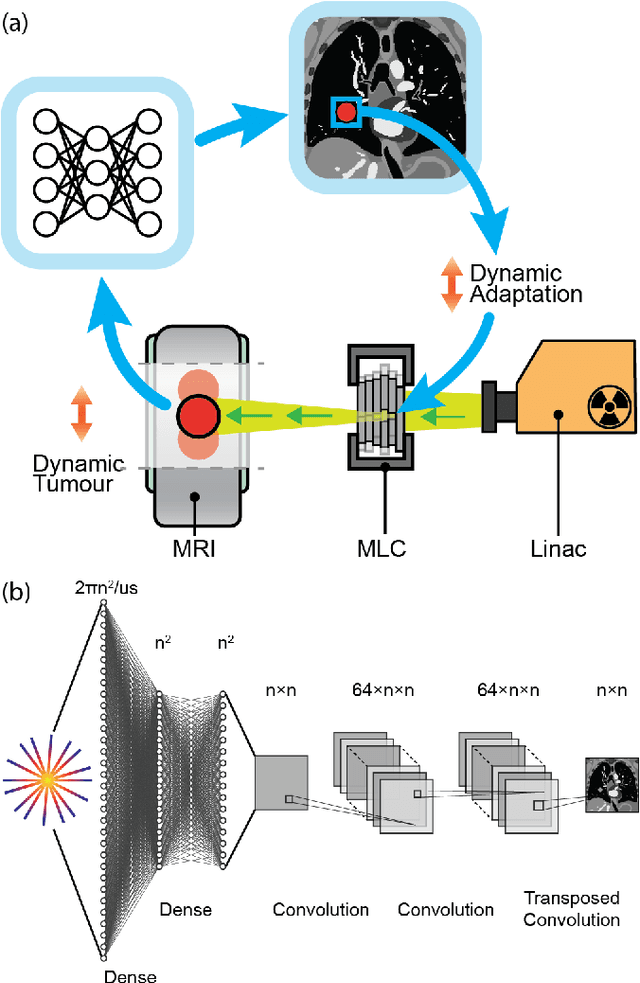
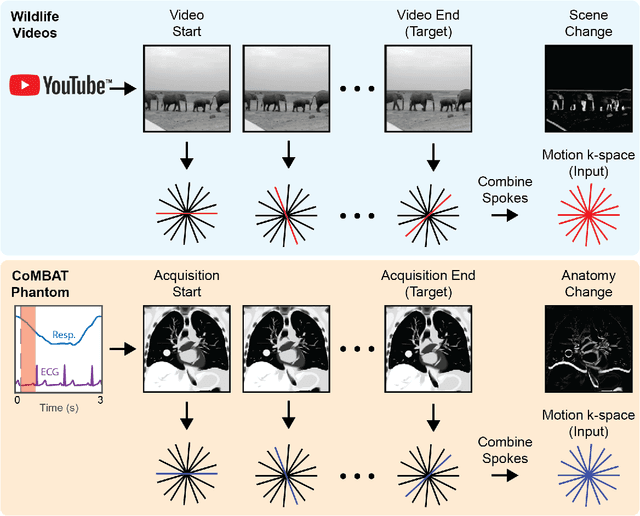
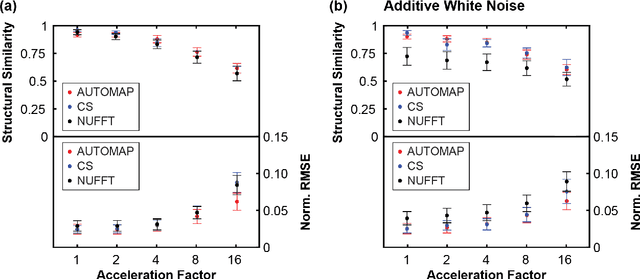
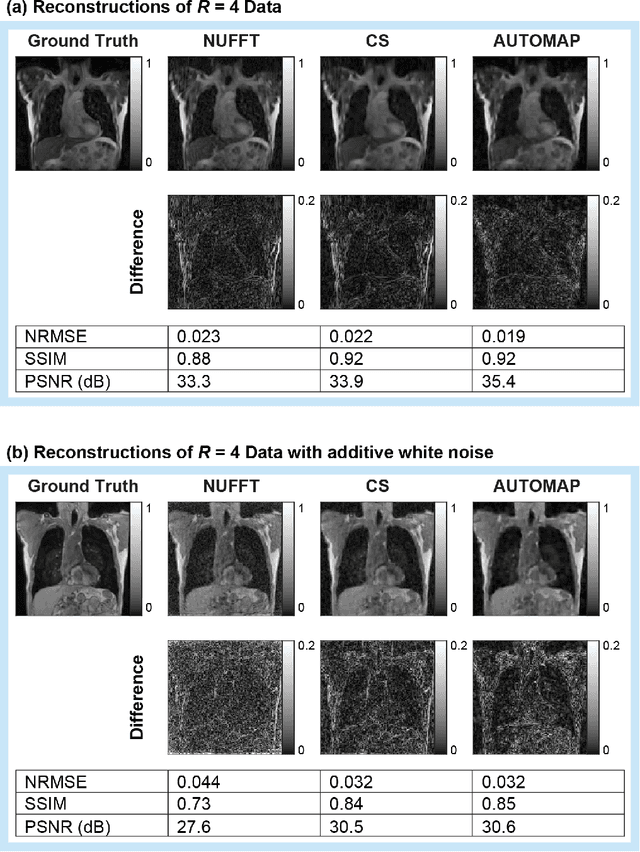
Abstract:MRI-guidance techniques that dynamically adapt radiation beams to follow tumor motion in real-time will lead to more accurate cancer treatments and reduced collateral healthy tissue damage. The gold-standard for reconstruction of undersampled MR data is compressed sensing (CS) which is computationally slow and limits the rate that images can be available for real-time adaptation. Here, we demonstrate the use of automated transform by manifold approximation (AUTOMAP), a generalized framework that maps raw MR signal to the target image domain, to rapidly reconstruct images from undersampled radial k-space data. The AUTOMAP neural network was trained to reconstruct images from a golden-angle radial acquisition, a benchmark for motion-sensitive imaging, on lung cancer patient data and generic images from ImageNet. Model training was subsequently augmented with motion-encoded k-space data derived from videos in the YouTube-8M dataset to encourage motion robust reconstruction. We find that AUTOMAP-reconstructed radial k-space has equivalent accuracy to CS but with much shorter processing times after initial fine-tuning on retrospectively acquired lung cancer patient data. Validation of motion-trained models with a virtual dynamic lung tumor phantom showed that the generalized motion properties learned from YouTube lead to improved target tracking accuracy. Our work shows that AUTOMAP can achieve real-time, accurate reconstruction of radial data. These findings imply that neural-network-based reconstruction is potentially superior to existing approaches for real-time image guidance applications.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge