Neeraj Wagh
Foundation Models for Brain Signals: A Critical Review of Current Progress and Future Directions
Jul 15, 2025Abstract:Patterns of electrical brain activity recorded via electroencephalography (EEG) offer immense value for scientific and clinical investigations. The inability of supervised EEG encoders to learn robust EEG patterns and their over-reliance on expensive signal annotations have sparked a transition towards general-purpose self-supervised EEG encoders, i.e., EEG foundation models (EEG-FMs), for robust and scalable EEG feature extraction. However, the real-world readiness of early EEG-FMs and the rubric for long-term research progress remain unclear. A systematic and comprehensive review of first-generation EEG-FMs is therefore necessary to understand the current state-of-the-art and identify key directions for future EEG-FMs. To that end, this study reviews 10 early EEG-FMs and presents a critical synthesis of their methodology, empirical findings, and outstanding research gaps. We find that most EEG-FMs adopt a sequence-based modeling scheme that relies on transformer-based backbones and the reconstruction of masked sequences for self-supervision. However, model evaluations remain heterogeneous and largely limited, making it challenging to assess their practical off-the-shelf utility. In addition to adopting standardized and realistic evaluations, future work should demonstrate more substantial scaling effects and make principled and trustworthy choices throughout the EEG representation learning pipeline. We believe that developing benchmarks, software tools, technical methodologies, and applications in collaboration with domain experts may further advance the translational utility and real-world adoption of EEG-FMs.
Tensor Decomposition of Large-scale Clinical EEGs Reveals Interpretable Patterns of Brain Physiology
Nov 24, 2022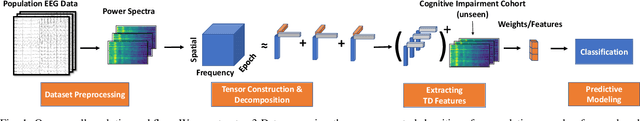
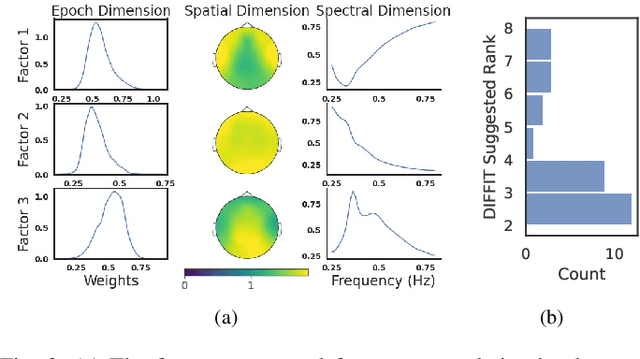
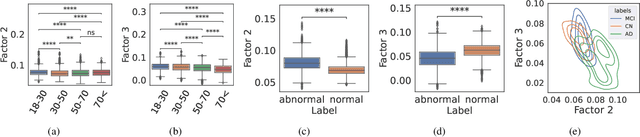
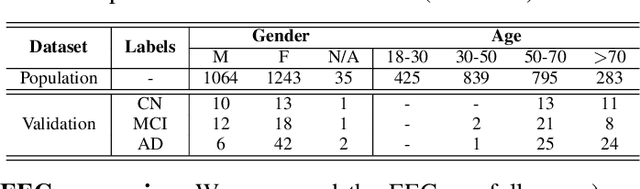
Abstract:Identifying abnormal patterns in electroencephalography (EEG) remains the cornerstone of diagnosing several neurological diseases. The current clinical EEG review process relies heavily on expert visual review, which is unscalable and error-prone. In an effort to augment the expert review process, there is a significant interest in mining population-level EEG patterns using unsupervised approaches. Current approaches rely either on two-dimensional decompositions (e.g., principal and independent component analyses) or deep representation learning (e.g., auto-encoders, self-supervision). However, most approaches do not leverage the natural multi-dimensional structure of EEGs and lack interpretability. In this study, we propose a tensor decomposition approach using the canonical polyadic decomposition to discover a parsimonious set of population-level EEG patterns, retaining the natural multi-dimensional structure of EEGs (time x space x frequency). We then validate their clinical value using a cohort of patients including varying stages of cognitive impairment. Our results show that the discovered patterns reflect physiologically meaningful features and accurately classify the stages of cognitive impairment (healthy vs mild cognitive impairment vs Alzheimer's dementia) with substantially fewer features compared to classical and deep learning-based baselines. We conclude that the decomposition of population-level EEG tensors recovers expert-interpretable EEG patterns that can aid in the study of smaller specialized clinical cohorts.
Assessing Robustness of EEG Representations under Data-shifts via Latent Space and Uncertainty Analysis
Sep 22, 2022
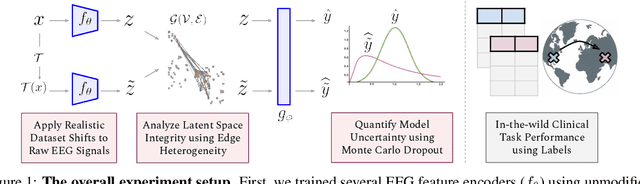
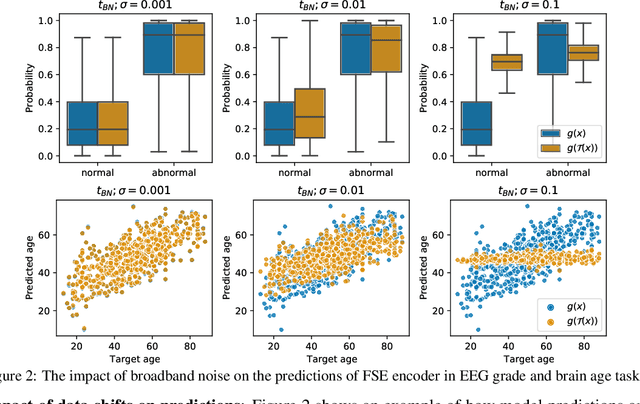
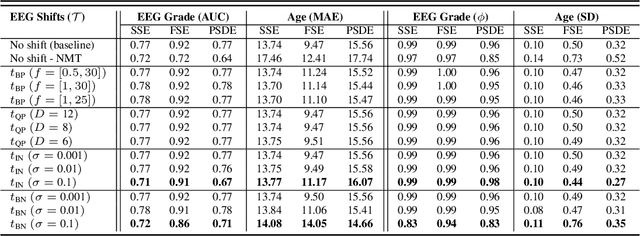
Abstract:The recent availability of large datasets in bio-medicine has inspired the development of representation learning methods for multiple healthcare applications. Despite advances in predictive performance, the clinical utility of such methods is limited when exposed to real-world data. Here we develop model diagnostic measures to detect potential pitfalls during deployment without assuming access to external data. Specifically, we focus on modeling realistic data shifts in electrophysiological signals (EEGs) via data transforms, and extend the conventional task-based evaluations with analyses of a) model's latent space and b) predictive uncertainty, under these transforms. We conduct experiments on multiple EEG feature encoders and two clinically relevant downstream tasks using publicly available large-scale clinical EEGs. Within this experimental setting, our results suggest that measures of latent space integrity and model uncertainty under the proposed data shifts may help anticipate performance degradation during deployment.
EEG-GCNN: Augmenting Electroencephalogram-based Neurological Disease Diagnosis using a Domain-guided Graph Convolutional Neural Network
Nov 17, 2020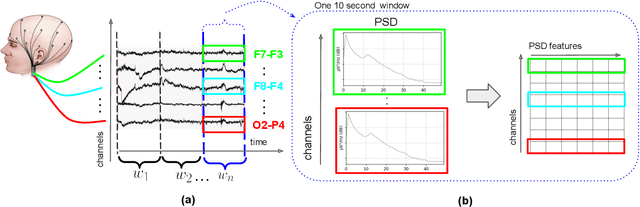

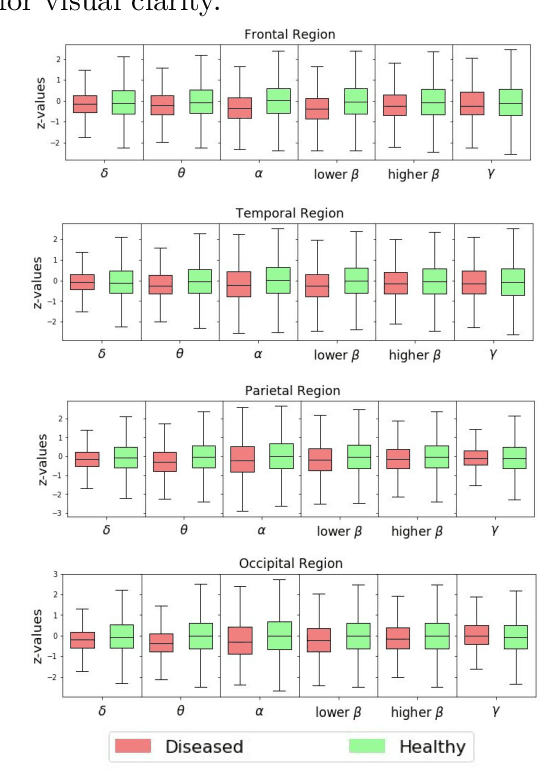

Abstract:This paper presents a novel graph convolutional neural network (GCNN)-based approach for improving the diagnosis of neurological diseases using scalp-electroencephalograms (EEGs). Although EEG is one of the main tests used for neurological-disease diagnosis, the sensitivity of EEG-based expert visual diagnosis remains at $\sim$50\%. This indicates a clear need for advanced methodology to reduce the false negative rate in detecting abnormal scalp-EEGs. In that context, we focus on the problem of distinguishing the abnormal scalp EEGs of patients with neurological diseases, which were originally classified as 'normal' by experts, from the scalp EEGs of healthy individuals. The contributions of this paper are three-fold: 1) we present EEG-GCNN, a novel GCNN model for EEG data that captures both the spatial and functional connectivity between the scalp electrodes, 2) using EEG-GCNN, we perform the first large-scale evaluation of the aforementioned hypothesis, and 3) using two large scalp-EEG databases, we demonstrate that EEG-GCNN significantly outperforms the human baseline and classical machine learning (ML) baselines, with an AUC of 0.90.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge