Nancy Leveson
Adverse Events in Robotic Surgery: A Retrospective Study of 14 Years of FDA Data
Jul 21, 2015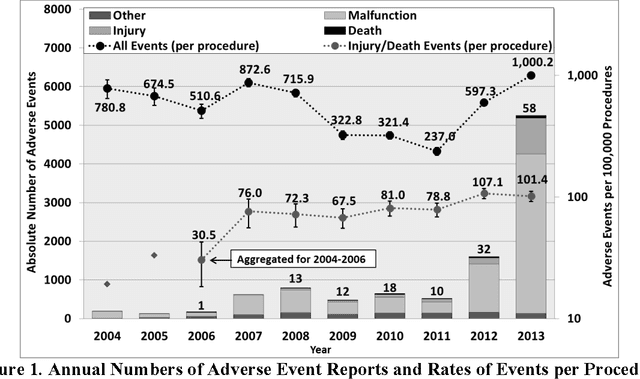
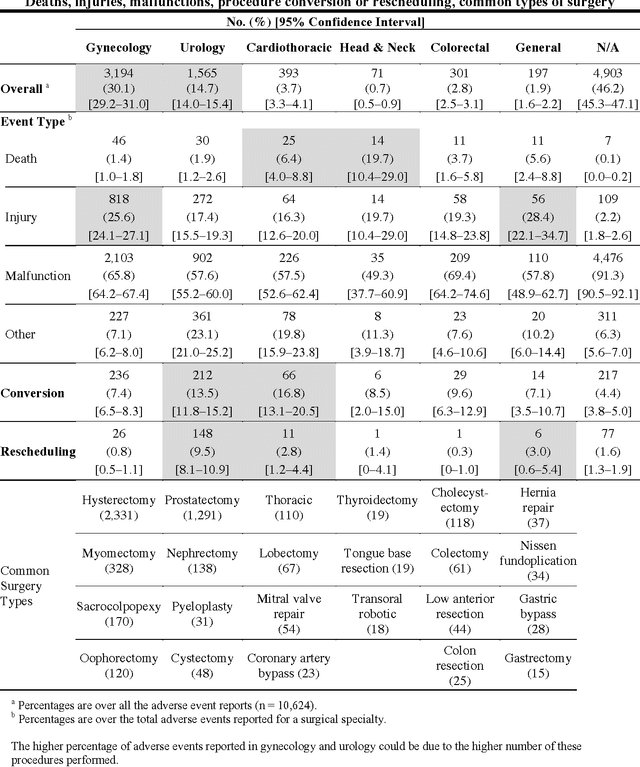
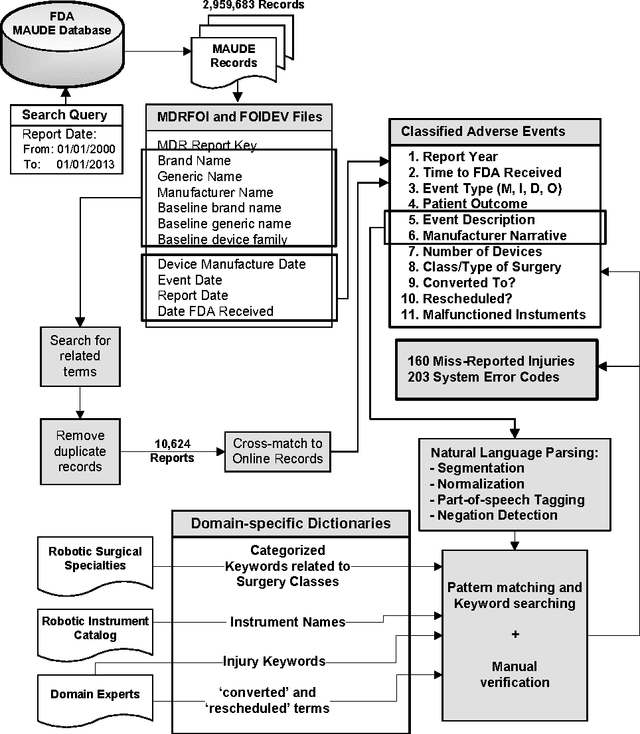
Abstract:Understanding the causes and patient impacts of surgical adverse events will help improve systems and operational practices to avoid incidents in the future. We analyzed the adverse events data related to robotic systems and instruments used in minimally invasive surgery, reported to the U.S. FDA MAUDE database from January 2000 to December 2013. We determined the number of events reported per procedure and per surgical specialty, the most common types of device malfunctions and their impact on patients, and the causes for catastrophic events such as major complications, patient injuries, and deaths. During the study period, 144 deaths (1.4% of the 10,624 reports), 1,391 patient injuries (13.1%), and 8,061 device malfunctions (75.9%) were reported. The numbers of injury and death events per procedure have stayed relatively constant since 2007 (mean = 83.4, 95% CI, 74.2-92.7). Surgical specialties, for which robots are extensively used, such as gynecology and urology, had lower number of injuries, deaths, and conversions per procedure than more complex surgeries, such as cardiothoracic and head and neck (106.3 vs. 232.9, Risk Ratio = 2.2, 95% CI, 1.9-2.6). Device and instrument malfunctions, such as falling of burnt/broken pieces of instruments into the patient (14.7%), electrical arcing of instruments (10.5%), unintended operation of instruments (8.6%), system errors (5%), and video/imaging problems (2.6%), constituted a major part of the reports. Device malfunctions impacted patients in terms of injuries or procedure interruptions. In 1,104 (10.4%) of the events, the procedure was interrupted to restart the system (3.1%), to convert the procedure to non-robotic techniques (7.3%), or to reschedule it to a later time (2.5%). Adoption of advanced techniques in design and operation of robotic surgical systems may reduce these preventable incidents in the future.
* Presented as the J. Maxwell Chamberlain Memorial Paper for adult cardiac surgery at the 50th Annual Meeting of the Society of Thoracic Surgeons in January. See Appendix for more detailed results, discussions, and related work. Updated the headers
Systems-theoretic Safety Assessment of Robotic Telesurgical Systems
Jul 08, 2015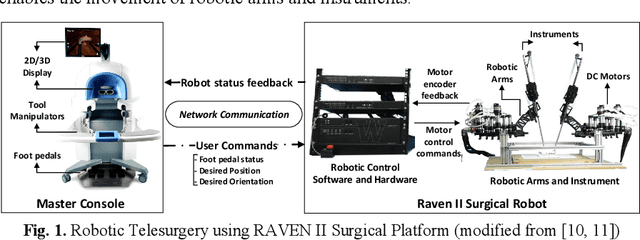
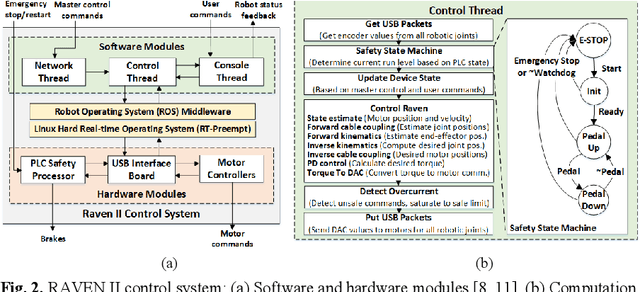
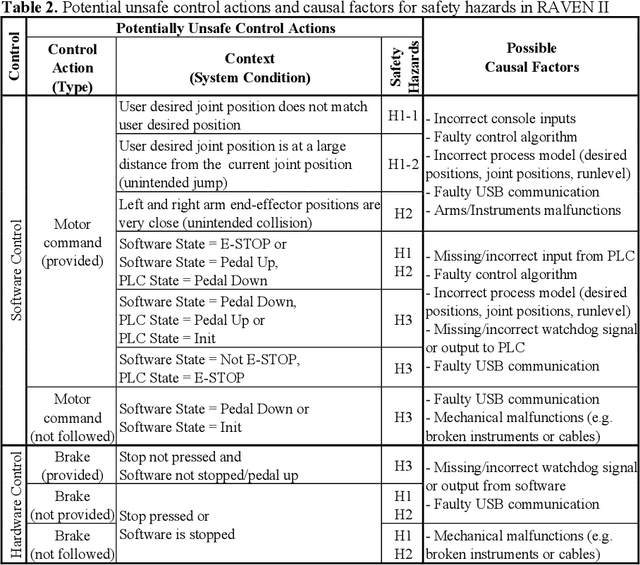
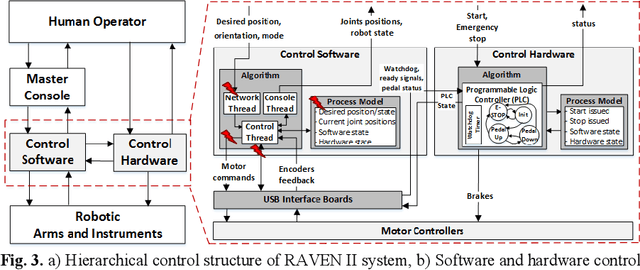
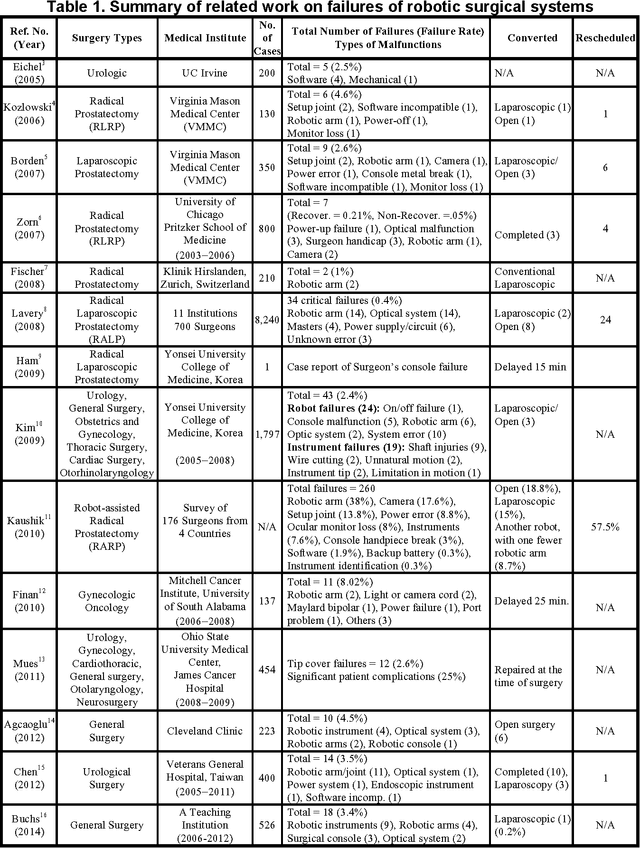
Abstract:Robotic telesurgical systems are one of the most complex medical cyber-physical systems on the market, and have been used in over 1.75 million procedures during the last decade. Despite significant improvements in design of robotic surgical systems through the years, there have been ongoing occurrences of safety incidents during procedures that negatively impact patients. This paper presents an approach for systems-theoretic safety assessment of robotic telesurgical systems using software-implemented fault-injection. We used a systemstheoretic hazard analysis technique (STPA) to identify the potential safety hazard scenarios and their contributing causes in RAVEN II robot, an open-source robotic surgical platform. We integrated the robot control software with a softwareimplemented fault-injection engine which measures the resilience of the system to the identified safety hazard scenarios by automatically inserting faults into different parts of the robot control software. Representative hazard scenarios from real robotic surgery incidents reported to the U.S. Food and Drug Administration (FDA) MAUDE database were used to demonstrate the feasibility of the proposed approach for safety-based design of robotic telesurgical systems.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge