Adverse Events in Robotic Surgery: A Retrospective Study of 14 Years of FDA Data
Paper and Code
Jul 21, 2015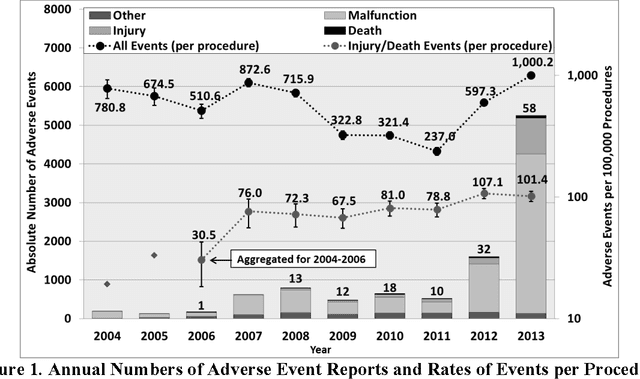
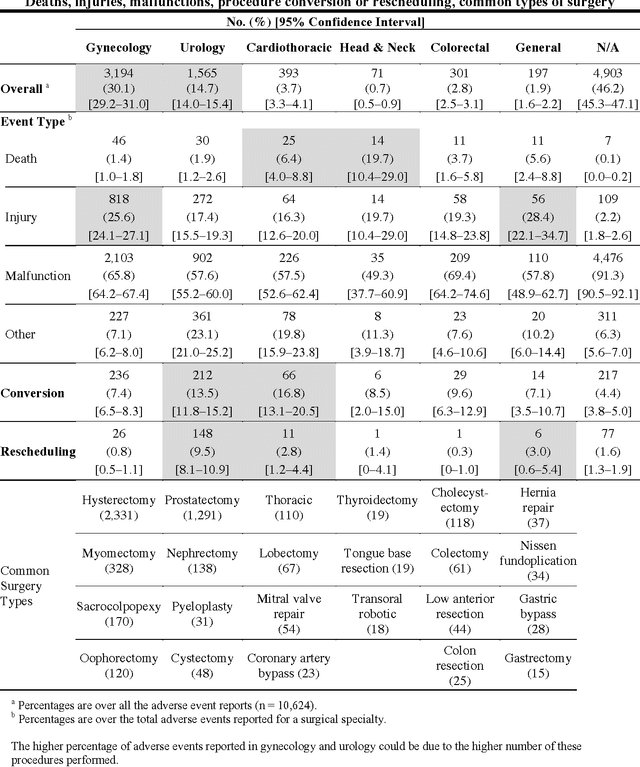
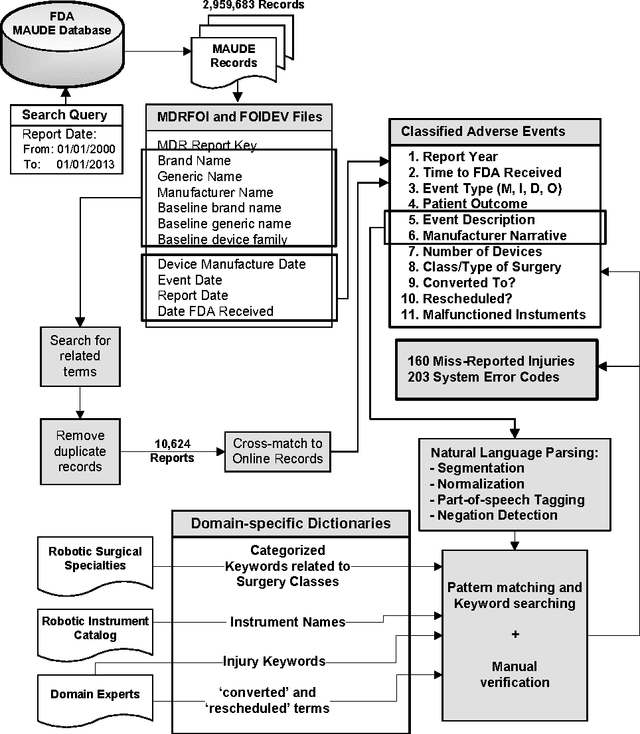
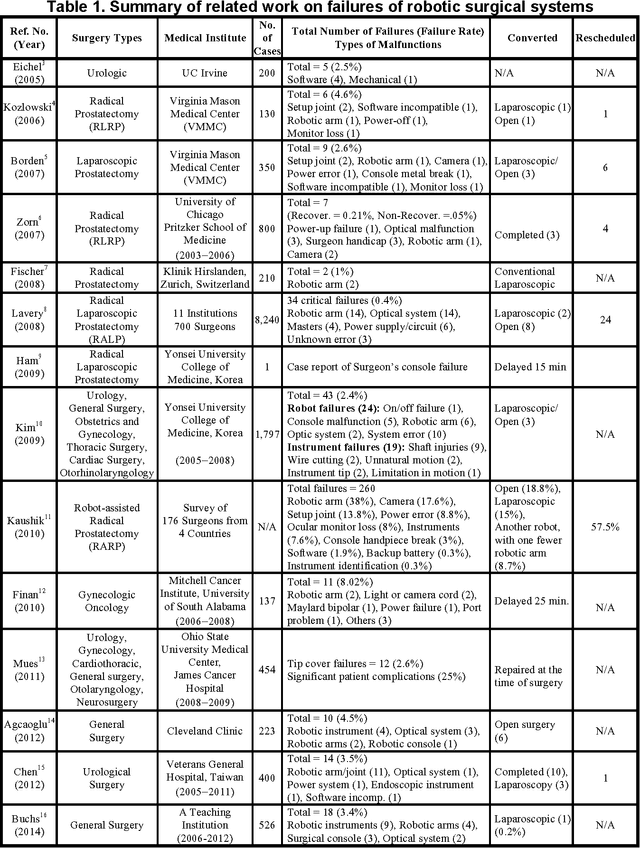
Understanding the causes and patient impacts of surgical adverse events will help improve systems and operational practices to avoid incidents in the future. We analyzed the adverse events data related to robotic systems and instruments used in minimally invasive surgery, reported to the U.S. FDA MAUDE database from January 2000 to December 2013. We determined the number of events reported per procedure and per surgical specialty, the most common types of device malfunctions and their impact on patients, and the causes for catastrophic events such as major complications, patient injuries, and deaths. During the study period, 144 deaths (1.4% of the 10,624 reports), 1,391 patient injuries (13.1%), and 8,061 device malfunctions (75.9%) were reported. The numbers of injury and death events per procedure have stayed relatively constant since 2007 (mean = 83.4, 95% CI, 74.2-92.7). Surgical specialties, for which robots are extensively used, such as gynecology and urology, had lower number of injuries, deaths, and conversions per procedure than more complex surgeries, such as cardiothoracic and head and neck (106.3 vs. 232.9, Risk Ratio = 2.2, 95% CI, 1.9-2.6). Device and instrument malfunctions, such as falling of burnt/broken pieces of instruments into the patient (14.7%), electrical arcing of instruments (10.5%), unintended operation of instruments (8.6%), system errors (5%), and video/imaging problems (2.6%), constituted a major part of the reports. Device malfunctions impacted patients in terms of injuries or procedure interruptions. In 1,104 (10.4%) of the events, the procedure was interrupted to restart the system (3.1%), to convert the procedure to non-robotic techniques (7.3%), or to reschedule it to a later time (2.5%). Adoption of advanced techniques in design and operation of robotic surgical systems may reduce these preventable incidents in the future.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge