Mostafa Fatehi
Guidelines and evaluation for clinical explainable AI on medical image analysis
Feb 16, 2022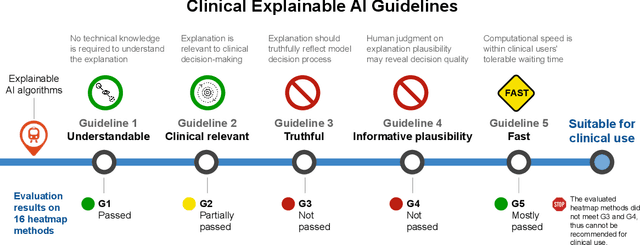
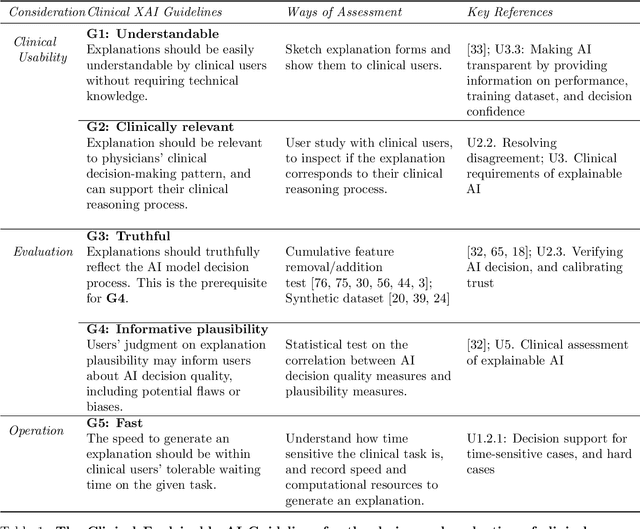
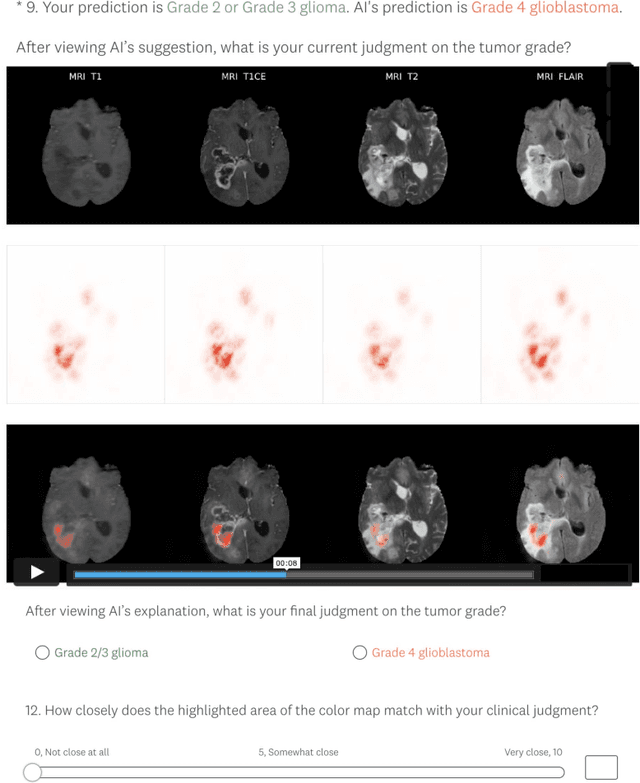
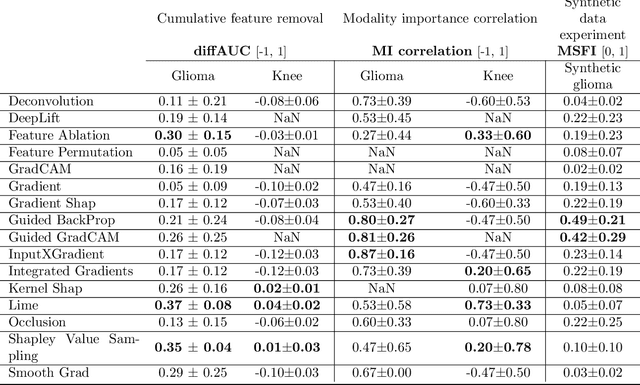
Abstract:Explainable artificial intelligence (XAI) is essential for enabling clinical users to get informed decision support from AI and comply with evidence-based medical practice. Applying XAI in clinical settings requires proper evaluation criteria to ensure the explanation technique is both technically sound and clinically useful, but specific support is lacking to achieve this goal. To bridge the research gap, we propose the Clinical XAI Guidelines that consist of five criteria a clinical XAI needs to be optimized for. The guidelines recommend choosing an explanation form based on Guideline 1 (G1) Understandability and G2 Clinical relevance. For the chosen explanation form, its specific XAI technique should be optimized for G3 Truthfulness, G4 Informative plausibility, and G5 Computational efficiency. Following the guidelines, we conducted a systematic evaluation on a novel problem of multi-modal medical image explanation with two clinical tasks, and proposed new evaluation metrics accordingly. The evaluated 16 commonly-used heatmap XAI techniques were not suitable for clinical use due to their failure in \textbf{G3} and \textbf{G4}. Our evaluation demonstrated the use of Clinical XAI Guidelines to support the design and evaluation for clinically viable XAI.
Applying Artificial Intelligence to Glioma Imaging: Advances and Challenges
Nov 28, 2019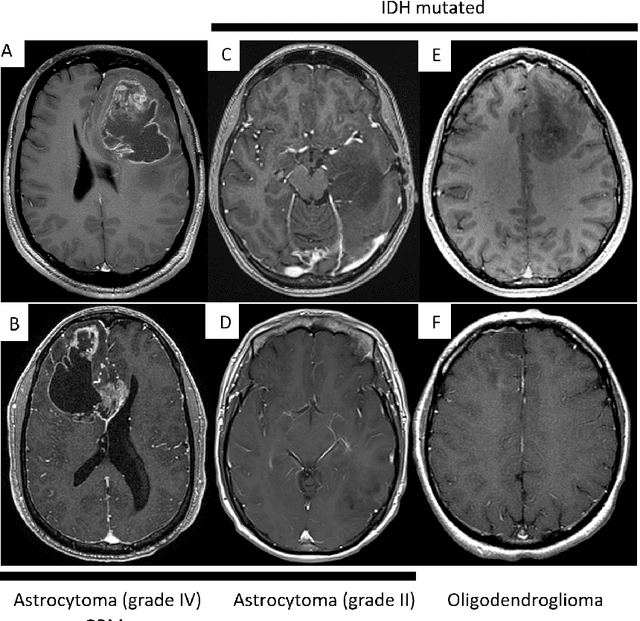
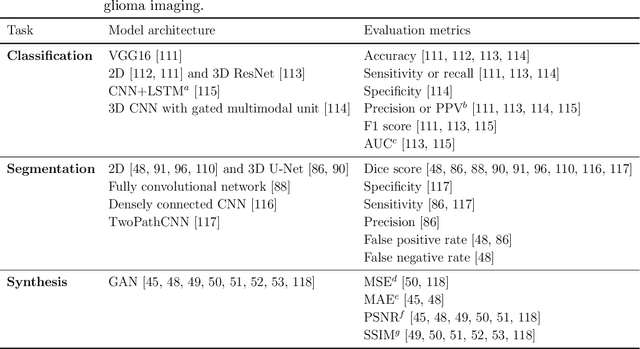
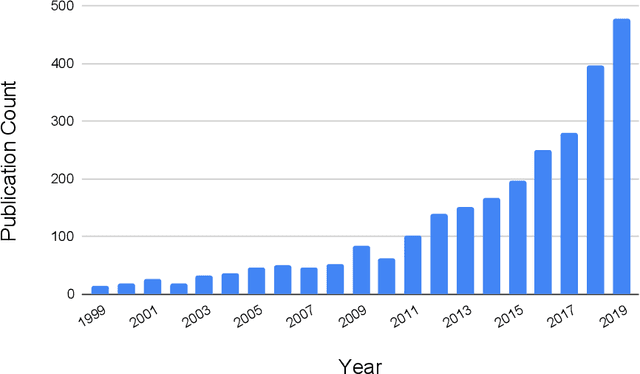
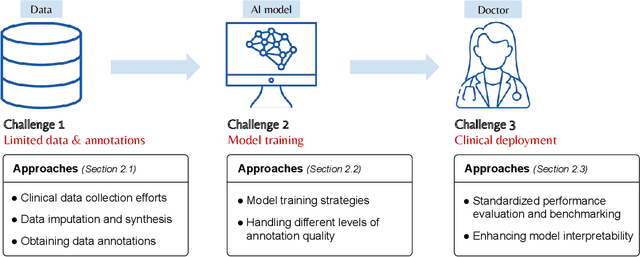
Abstract:Primary brain tumors including gliomas continue to pose significant management challenges to clinicians. While the presentation, the pathology, and the clinical course of these lesions is variable, the initial investigations are usually similar. Patients who are suspected to have a brain tumor will be assessed with computed tomography (CT) and magnetic resonance imaging (MRI). The imaging findings are used by neurosurgeons to determine the feasibility of surgical resection and plan such an undertaking. Imaging studies are also an indispensable tool in tracking tumor progression or its response to treatment. As these imaging studies are non-invasive, relatively cheap and accessible to patients, there have been many efforts over the past two decades to increase the amount of clinically-relevant information that can be extracted from brain imaging. Most recently, artificial intelligence (AI) techniques have been employed to segment and characterize brain tumors, as well as to detect progression or treatment-response. However, the clinical utility of such endeavours remains limited due to challenges in data collection and annotation, model training, and in the reliability of AI-generated information. We provide a review of recent advances in addressing the above challenges. First, to overcome the challenge of data paucity, different image imputation and synthesis techniques along with annotation collection efforts are summarized. Next, various training strategies are presented to meet multiple desiderata, such as model performance, generalization ability, data privacy protection, and learning with sparse annotations. Finally, standardized performance evaluation and model interpretability methods have been reviewed. We believe that these technical approaches will facilitate the development of a fully-functional AI tool in the clinical care of patients with gliomas.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge