Mina Jafari
A Decision Making Approach for Chemotherapy Planning based on Evolutionary Processing
Mar 19, 2023



Abstract:The problem of chemotherapy treatment optimization can be defined in order to minimize the size of the tumor without endangering the patient's health; therefore, chemotherapy requires to achieve a number of objectives, simultaneously. For this reason, the optimization problem turns to a multi-objective problem. In this paper, a multi-objective meta-heuristic method is provided for cancer chemotherapy with the aim of balancing between two objectives: the amount of toxicity and the number of cancerous cells. The proposed method uses mathematical models in order to measure the drug concentration, tumor growth and the amount of toxicity. This method utilizes a Multi-Objective Particle Swarm Optimization (MOPSO) algorithm to optimize cancer chemotherapy plan using cell-cycle specific drugs. The proposed method can be a good model for personalized medicine as it returns a set of solutions as output that have balanced between different objectives and provided the possibility to choose the most appropriate therapeutic plan based on some information about the status of the patient. Experimental results confirm that the proposed method is able to explore the search space efficiently in order to find out the suitable treatment plan with minimal side effects. This main objective is provided using a desirable designing of chemotherapy drugs and controlling the injection dose. Moreover, results show that the proposed method achieve to a better therapeutic performance compared to a more recent similar method [1].
FU-net: Multi-class Image Segmentation Using Feedback Weighted U-net
Apr 28, 2020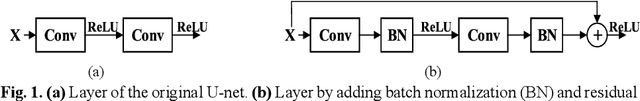
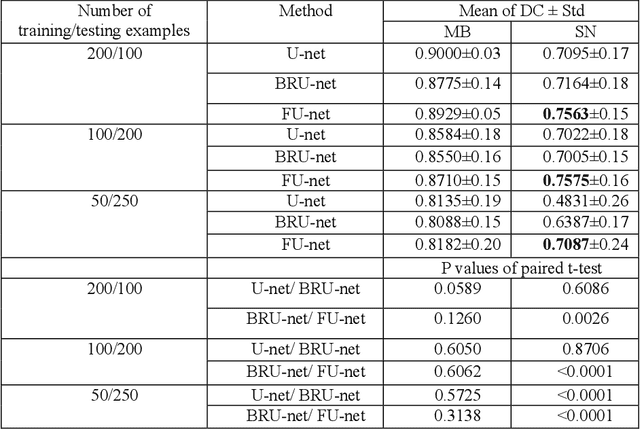

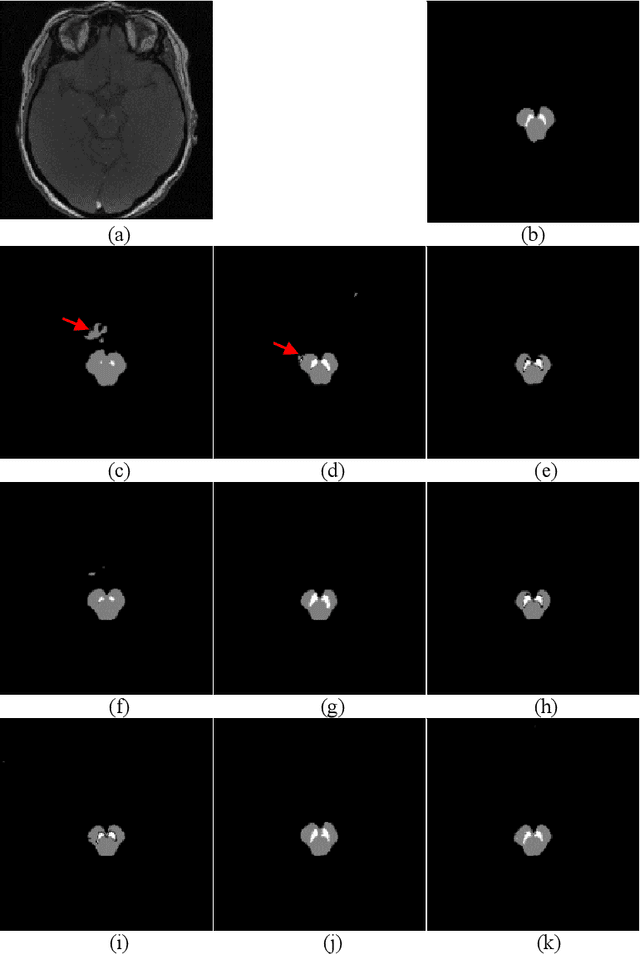
Abstract:In this paper, we present a generic deep convolutional neural network (DCNN) for multi-class image segmentation. It is based on a well-established supervised end-to-end DCNN model, known as U-net. U-net is firstly modified by adding widely used batch normalization and residual block (named as BRU-net) to improve the efficiency of model training. Based on BRU-net, we further introduce a dynamically weighted cross-entropy loss function. The weighting scheme is calculated based on the pixel-wise prediction accuracy during the training process. Assigning higher weights to pixels with lower segmentation accuracies enables the network to learn more from poorly predicted image regions. Our method is named as feedback weighted U-net (FU-net). We have evaluated our method based on T1- weighted brain MRI for the segmentation of midbrain and substantia nigra, where the number of pixels in each class is extremely unbalanced to each other. Based on the dice coefficient measurement, our proposed FU-net has outperformed BRU-net and U-net with statistical significance, especially when only a small number of training examples are available. The code is publicly available in GitHub (GitHub link: https://github.com/MinaJf/FU-net).
* Accepted for publication at International Conference on Image and Graphics (ICIG 2019)
DRU-net: An Efficient Deep Convolutional Neural Network for Medical Image Segmentation
Apr 28, 2020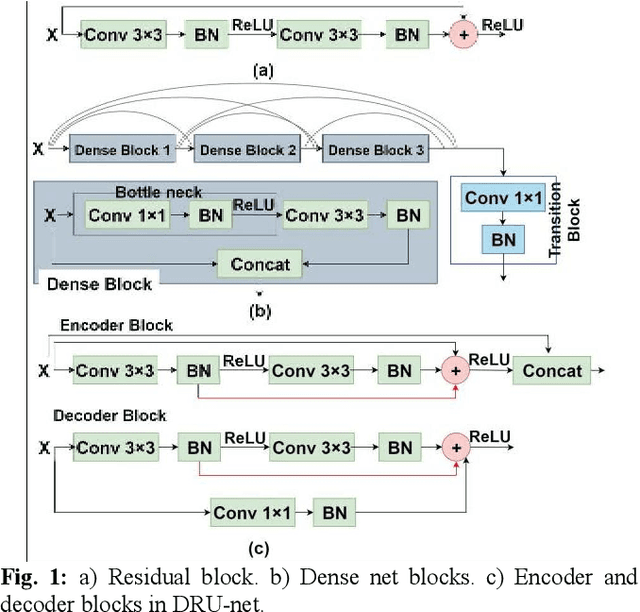
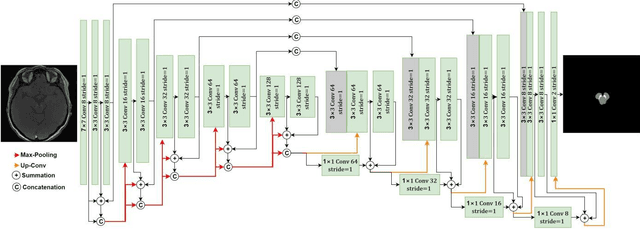
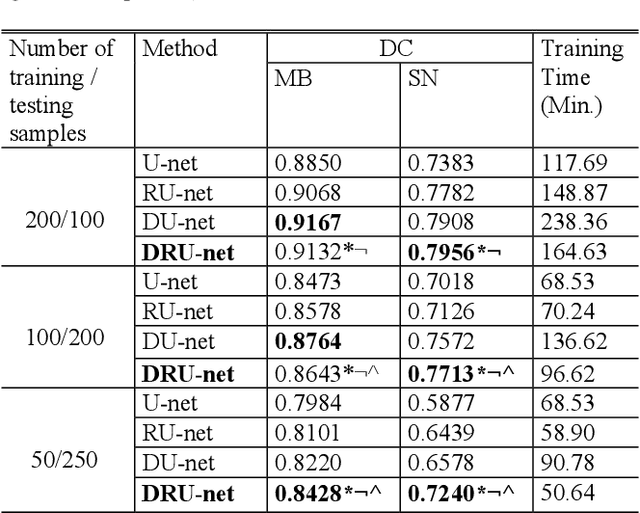
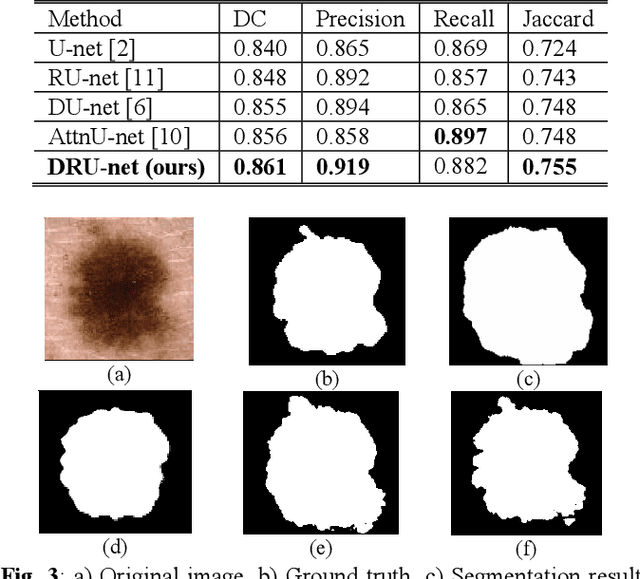
Abstract:Residual network (ResNet) and densely connected network (DenseNet) have significantly improved the training efficiency and performance of deep convolutional neural networks (DCNNs) mainly for object classification tasks. In this paper, we propose an efficient network architecture by considering advantages of both networks. The proposed method is integrated into an encoder-decoder DCNN model for medical image segmentation. Our method adds additional skip connections compared to ResNet but uses significantly fewer model parameters than DenseNet. We evaluate the proposed method on a public dataset (ISIC 2018 grand-challenge) for skin lesion segmentation and a local brain MRI dataset. In comparison with ResNet-based, DenseNet-based and attention network (AttnNet) based methods within the same encoder-decoder network structure, our method achieves significantly higher segmentation accuracy with fewer number of model parameters than DenseNet and AttnNet. The code is available on GitHub (GitHub link: https://github.com/MinaJf/DRU-net).
* Accepted for publication at IEEE International Symposium on Biomedical Imaging (ISBI) 2020, 5 pages, 3 figures
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge