Michal Kazmierski
Lymph Node Graph Neural Networks for Cancer Metastasis Prediction
Jun 03, 2021



Abstract:Predicting outcomes, such as survival or metastasis for individual cancer patients is a crucial component of precision oncology. Machine learning (ML) offers a promising way to exploit rich multi-modal data, including clinical information and imaging to learn predictors of disease trajectory and help inform clinical decision making. In this paper, we present a novel graph-based approach to incorporate imaging characteristics of existing cancer spread to local lymph nodes (LNs) as well as their connectivity patterns in a prognostic ML model. We trained an edge-gated Graph Convolutional Network (Gated-GCN) to accurately predict the risk of distant metastasis (DM) by propagating information across the LN graph with the aid of soft edge attention mechanism. In a cohort of 1570 head and neck cancer patients, the Gated-GCN achieves AUROC of 0.757 for 2-year DM classification and $C$-index of 0.725 for lifetime DM risk prediction, outperforming current prognostic factors as well as previous approaches based on aggregated LN features. We also explored the importance of graph structure and individual lymph nodes through ablation experiments and interpretability studies, highlighting the importance of considering individual LN characteristics as well as the relationships between regions of cancer spread.
A Machine Learning Challenge for Prognostic Modelling in Head and Neck Cancer Using Multi-modal Data
Jan 28, 2021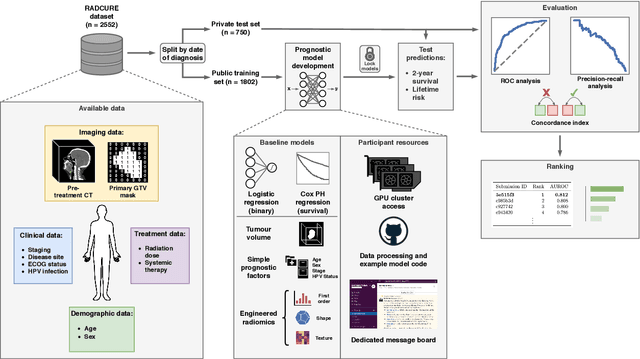

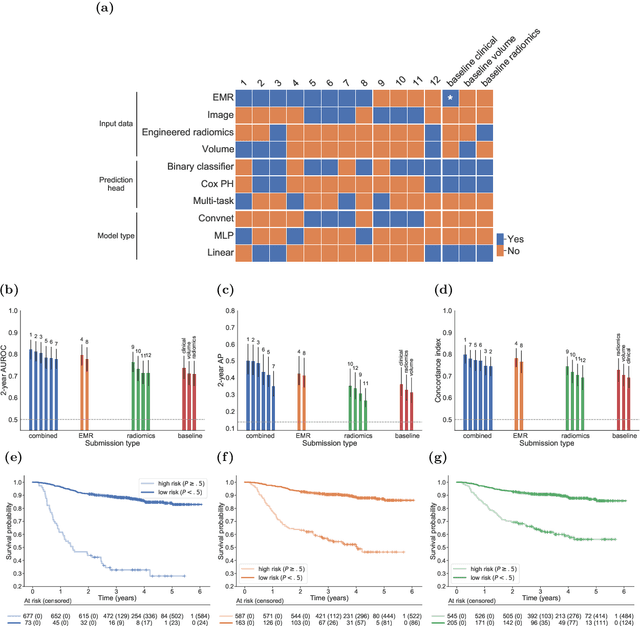
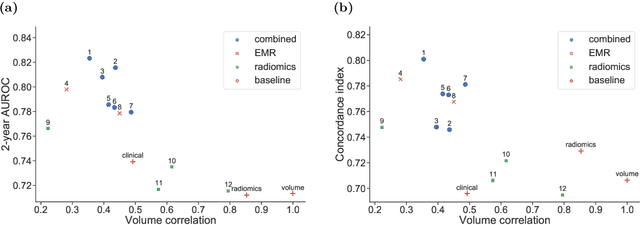
Abstract:Accurate prognosis for an individual patient is a key component of precision oncology. Recent advances in machine learning have enabled the development of models using a wider range of data, including imaging. Radiomics aims to extract quantitative predictive and prognostic biomarkers from routine medical imaging, but evidence for computed tomography radiomics for prognosis remains inconclusive. We have conducted an institutional machine learning challenge to develop an accurate model for overall survival prediction in head and neck cancer using clinical data etxracted from electronic medical records and pre-treatment radiological images, as well as to evaluate the true added benefit of radiomics for head and neck cancer prognosis. Using a large, retrospective dataset of 2,552 patients and a rigorous evaluation framework, we compared 12 different submissions using imaging and clinical data, separately or in combination. The winning approach used non-linear, multitask learning on clinical data and tumour volume, achieving high prognostic accuracy for 2-year and lifetime survival prediction and outperforming models relying on clinical data only, engineered radiomics and deep learning. Combining all submissions in an ensemble model resulted in improved accuracy, with the highest gain from a image-based deep learning model. Our results show the potential of machine learning and simple, informative prognostic factors in combination with large datasets as a tool to guide personalized cancer care.
Deep-CR MTLR: a Multi-Modal Approach for Cancer Survival Prediction with Competing Risks
Dec 10, 2020


Abstract:Accurate survival prediction is crucial for development of precision cancer medicine, creating the need for new sources of prognostic information. Recently, there has been significant interest in exploiting routinely collected clinical and medical imaging data to discover new prognostic markers in multiple cancer types. However, most of the previous studies focus on individual data modalities alone and do not make use of recent advances in machine learning for survival prediction. We present Deep-CR MTLR -- a novel machine learning approach for accurate cancer survival prediction from multi-modal clinical and imaging data in the presence of competing risks based on neural networks and an extension of the multi-task logistic regression framework. We demonstrate improved prognostic performance of the multi-modal approach over single modality predictors in a cohort of 2552 head and neck cancer patients, particularly for cancer specific survival, where our approach achieves 2-year AUROC of 0.774 and $C$-index of 0.788.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge