Michael Reutlinger
Drug Discovery under Covariate Shift with Domain-Informed Prior Distributions over Functions
Jul 14, 2023



Abstract:Accelerating the discovery of novel and more effective therapeutics is an important pharmaceutical problem in which deep learning is playing an increasingly significant role. However, real-world drug discovery tasks are often characterized by a scarcity of labeled data and significant covariate shift$\unicode{x2013}\unicode{x2013}$a setting that poses a challenge to standard deep learning methods. In this paper, we present Q-SAVI, a probabilistic model able to address these challenges by encoding explicit prior knowledge of the data-generating process into a prior distribution over functions, presenting researchers with a transparent and probabilistically principled way to encode data-driven modeling preferences. Building on a novel, gold-standard bioactivity dataset that facilitates a meaningful comparison of models in an extrapolative regime, we explore different approaches to induce data shift and construct a challenging evaluation setup. We then demonstrate that using Q-SAVI to integrate contextualized prior knowledge of drug-like chemical space into the modeling process affords substantial gains in predictive accuracy and calibration, outperforming a broad range of state-of-the-art self-supervised pre-training and domain adaptation techniques.
Benchmarking Accuracy and Generalizability of Four Graph Neural Networks Using Large In Vitro ADME Datasets from Different Chemical Spaces
Nov 27, 2021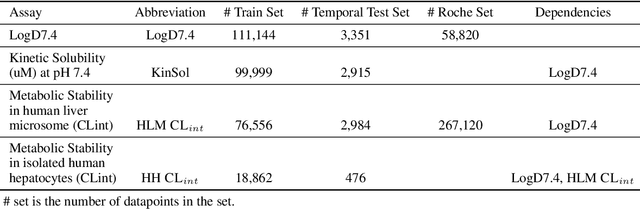
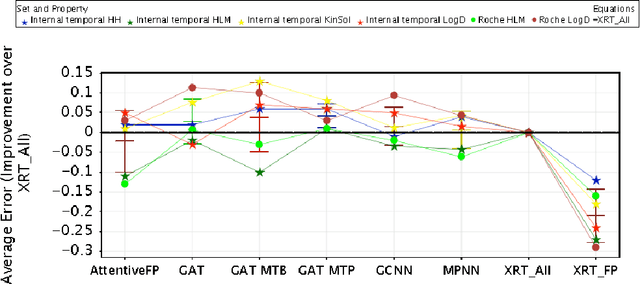
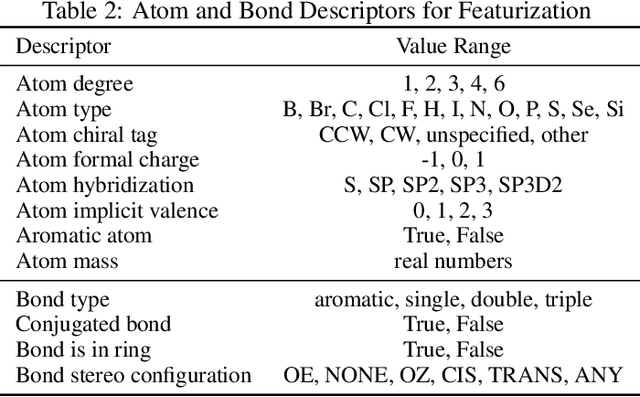
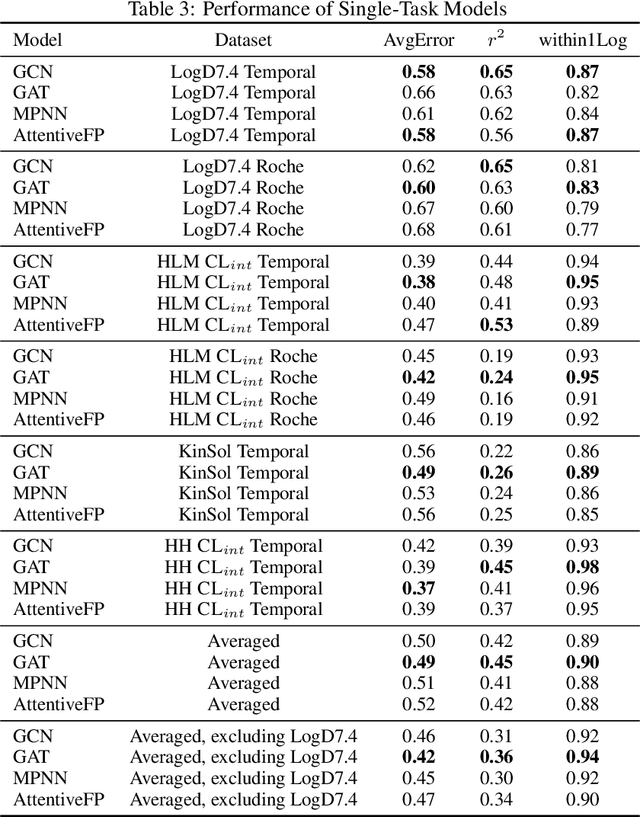
Abstract:In this work, we benchmark a variety of single- and multi-task graph neural network (GNN) models against lower-bar and higher-bar traditional machine learning approaches employing human engineered molecular features. We consider four GNN variants -- Graph Convolutional Network (GCN), Graph Attention Network (GAT), Message Passing Neural Network (MPNN), and Attentive Fingerprint (AttentiveFP). So far deep learning models have been primarily benchmarked using lower-bar traditional models solely based on fingerprints, while more realistic benchmarks employing fingerprints, whole-molecule descriptors and predictions from other related endpoints (e.g., LogD7.4) appear to be scarce for industrial ADME datasets. In addition to time-split test sets based on Genentech data, this study benefits from the availability of measurements from an external chemical space (Roche data). We identify GAT as a promising approach to implementing deep learning models. While all GNN models significantly outperform lower-bar benchmark traditional models solely based on fingerprints, only GATs seem to offer a small but consistent improvement over higher-bar benchmark traditional models. Finally, the accuracy of in vitro assays from different laboratories predicting the same experimental endpoints appears to be comparable with the accuracy of GAT single-task models, suggesting that most of the observed error from the models is a function of the experimental error propagation.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge