Megan Schuurmans
Navigating the landscape of multimodal AI in medicine: a scoping review on technical challenges and clinical applications
Nov 06, 2024
Abstract:Recent technological advances in healthcare have led to unprecedented growth in patient data quantity and diversity. While artificial intelligence (AI) models have shown promising results in analyzing individual data modalities, there is increasing recognition that models integrating multiple complementary data sources, so-called multimodal AI, could enhance clinical decision-making. This scoping review examines the landscape of deep learning-based multimodal AI applications across the medical domain, analyzing 432 papers published between 2018 and 2024. We provide an extensive overview of multimodal AI development across different medical disciplines, examining various architectural approaches, fusion strategies, and common application areas. Our analysis reveals that multimodal AI models consistently outperform their unimodal counterparts, with an average improvement of 6.2 percentage points in AUC. However, several challenges persist, including cross-departmental coordination, heterogeneous data characteristics, and incomplete datasets. We critically assess the technical and practical challenges in developing multimodal AI systems and discuss potential strategies for their clinical implementation, including a brief overview of commercially available multimodal AI models for clinical decision-making. Additionally, we identify key factors driving multimodal AI development and propose recommendations to accelerate the field's maturation. This review provides researchers and clinicians with a thorough understanding of the current state, challenges, and future directions of multimodal AI in medicine.
Fully Automatic Deep Learning Framework for Pancreatic Ductal Adenocarcinoma Detection on Computed Tomography
Dec 02, 2021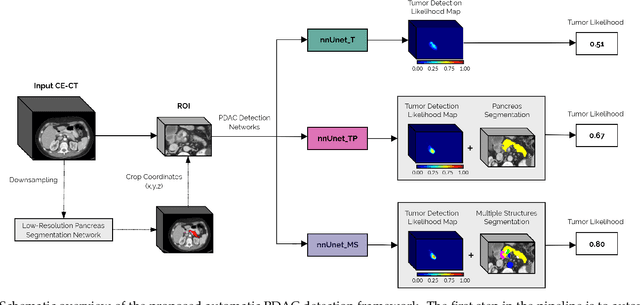

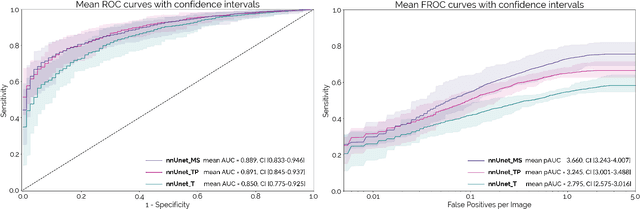
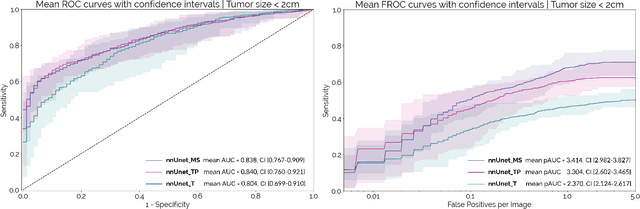
Abstract:Early detection improves prognosis in pancreatic ductal adenocarcinoma (PDAC) but is challenging as lesions are often small and poorly defined on contrast-enhanced computed tomography scans (CE-CT). Deep learning can facilitate PDAC diagnosis, however current models still fail to identify small (<2cm) lesions. In this study, state-of-the-art deep learning models were used to develop an automatic framework for PDAC detection, focusing on small lesions. Additionally, the impact of integrating surrounding anatomy was investigated. CE-CT scans from a cohort of 119 pathology-proven PDAC patients and a cohort of 123 patients without PDAC were used to train a nnUnet for automatic lesion detection and segmentation (nnUnet_T). Two additional nnUnets were trained to investigate the impact of anatomy integration: (1) segmenting the pancreas and tumor (nnUnet_TP), (2) segmenting the pancreas, tumor, and multiple surrounding anatomical structures (nnUnet_MS). An external, publicly available test set was used to compare the performance of the three networks. The nnUnet_MS achieved the best performance, with an area under the receiver operating characteristic curve of 0.91 for the whole test set and 0.88 for tumors <2cm, showing that state-of-the-art deep learning can detect small PDAC and benefits from anatomy information.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge