Lihong Ma
The Joint Entity-Relation Extraction Model Based on Span and Interactive Fusion Representation for Chinese Medical Texts with Complex Semantics
Feb 13, 2025Abstract:Joint entity-relation extraction is a critical task in transforming unstructured or semi-structured text into triplets, facilitating the construction of large-scale knowledge graphs, and supporting various downstream applications. Despite its importance, research on Chinese text, particularly with complex semantics in specialized domains like medicine, remains limited. To address this gap, we introduce the CH-DDI, a Chinese drug-drug interactions dataset designed to capture the intricacies of medical text. Leveraging the strengths of attention mechanisms in capturing long-range dependencies, we propose the SEA module, which enhances the extraction of complex contextual semantic information, thereby improving entity recognition and relation extraction. Additionally, to address the inefficiencies of existing methods in facilitating information exchange between entity recognition and relation extraction, we present an interactive fusion representation module. This module employs Cross Attention for bidirectional information exchange between the tasks and further refines feature extraction through BiLSTM. Experimental results on both our CH-DDI dataset and public CoNLL04 dataset demonstrate that our model exhibits strong generalization capabilities. On the CH-DDI dataset, our model achieves an F1-score of 96.73% for entity recognition and 78.43% for relation extraction. On the CoNLL04 dataset, it attains an entity recognition precision of 89.54% and a relation extraction accuracy of 71.64%.
Static and multivariate-temporal attentive fusion transformer for readmission risk prediction
Jul 15, 2024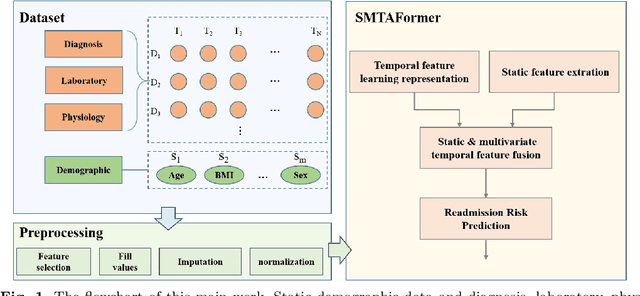
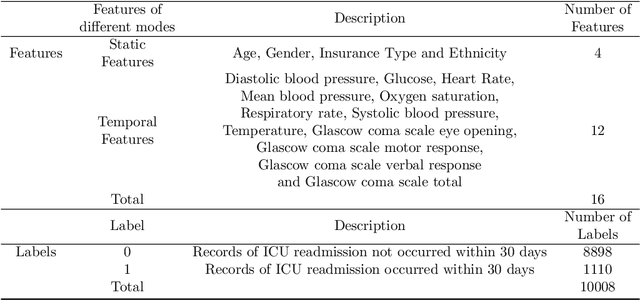
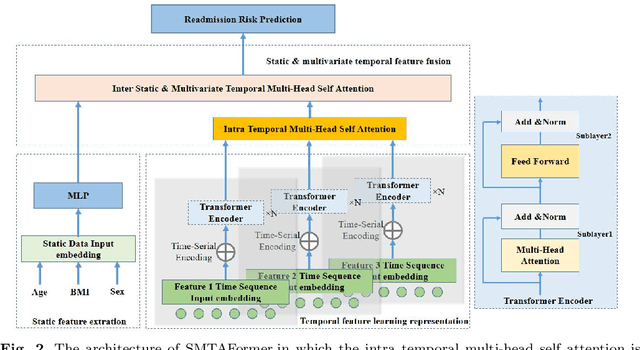

Abstract:Background: Accurate short-term readmission prediction of ICU patients is significant in improving the efficiency of resource assignment by assisting physicians in making discharge decisions. Clinically, both individual static static and multivariate temporal data collected from ICU monitors play critical roles in short-term readmission prediction. Informative static and multivariate temporal feature representation capturing and fusion present challenges for accurate readmission prediction. Methods:We propose a novel static and multivariate-temporal attentive fusion transformer (SMTAFormer) to predict short-term readmission of ICU patients by fully leveraging the potential of demographic and dynamic temporal data. In SMTAFormer, we first apply an MLP network and a temporal transformer network to learn useful static and temporal feature representations, respectively. Then, the well-designed static and multivariate temporal feature fusion module is applied to fuse static and temporal feature representations by modeling intra-correlation among multivariate temporal features and constructing inter-correlation between static and multivariate temporal features. Results: We construct a readmission risk assessment (RRA) dataset based on the MIMIC-III dataset. The extensive experiments show that SMTAFormer outperforms advanced methods, in which the accuracy of our proposed method is up to 86.6%, and the area under the receiver operating characteristic curve (AUC) is up to 0.717. Conclusion: Our proposed SMTAFormer can efficiently capture and fuse static and multivariate temporal feature representations. The results show that SMTAFormer significantly improves the short-term readmission prediction performance of ICU patients through comparisons to strong baselines.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge