Katja Pinker
Predicting breast cancer with AI for individual risk-adjusted MRI screening and early detection
Nov 29, 2023Abstract:Women with an increased life-time risk of breast cancer undergo supplemental annual screening MRI. We propose to predict the risk of developing breast cancer within one year based on the current MRI, with the objective of reducing screening burden and facilitating early detection. An AI algorithm was developed on 53,858 breasts from 12,694 patients who underwent screening or diagnostic MRI and accrued over 12 years, with 2,331 confirmed cancers. A first U-Net was trained to segment lesions and identify regions of concern. A second convolutional network was trained to detect malignant cancer using features extracted by the U-Net. This network was then fine-tuned to estimate the risk of developing cancer within a year in cases that radiologists considered normal or likely benign. Risk predictions from this AI were evaluated with a retrospective analysis of 9,183 breasts from a high-risk screening cohort, which were not used for training. Statistical analysis focused on the tradeoff between number of omitted exams versus negative predictive value, and number of potential early detections versus positive predictive value. The AI algorithm identified regions of concern that coincided with future tumors in 52% of screen-detected cancers. Upon directed review, a radiologist found that 71.3% of cancers had a visible correlate on the MRI prior to diagnosis, 65% of these correlates were identified by the AI model. Reevaluating these regions in 10% of all cases with higher AI-predicted risk could have resulted in up to 33% early detections by a radiologist. Additionally, screening burden could have been reduced in 16% of lower-risk cases by recommending a later follow-up without compromising current interval cancer rate. With increasing datasets and improving image quality we expect this new AI-aided, adaptive screening to meaningfully reduce screening burden and improve early detection.
Deep learning achieves radiologist-level performance of tumor segmentation in breast MRI
Sep 21, 2020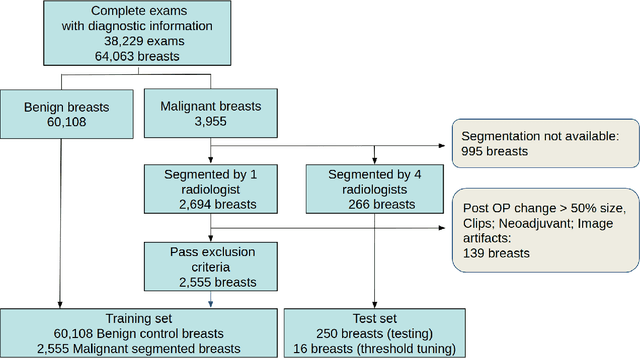
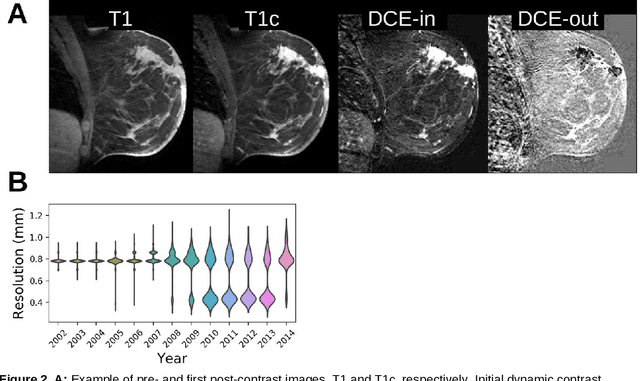
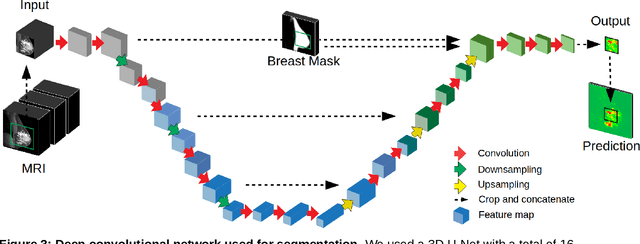
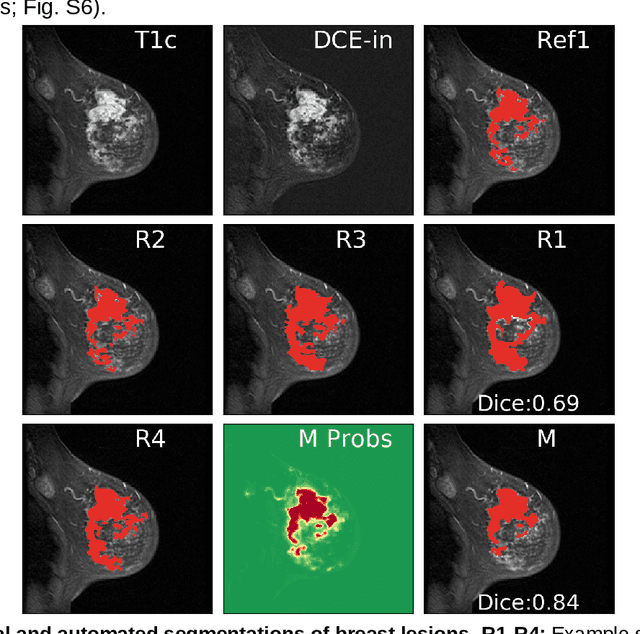
Abstract:Purpose: The goal of this research was to develop a deep network architecture that achieves fully-automated radiologist-level segmentation of breast tumors in MRI. Materials and Methods: We leveraged 38,229 clinical MRI breast exams collected retrospectively from women aged 12-94 (mean age 54) who presented between 2002 and 2014 at a single clinical site. The training set for the network consisted of 2,555 malignant breasts that were segmented in 2D by experienced radiologists, as well as 60,108 benign breasts that served as negative controls. The test set consisted of 250 exams with tumors segmented independently by four radiologists. We selected among several 3D deep convolutional neural network architectures, input modalities and harmonization methods. The outcome measure was the Dice score for 2D segmentation, and was compared between the network and radiologists using the Wilcoxon signed-rank test and the TOST procedure. Results: The best-performing network on the training set was a volumetric U-Net with contrast enhancement dynamic as input and with intensity normalized for each exam. In the test set the median Dice score of this network was 0.77. The performance of the network was equivalent to that of the radiologists (TOST procedure with radiologist performance of 0.69-0.84 as equivalence bounds: p = 5e-10 and p = 2e-5, respectively; N = 250) and compares favorably with published state of the art (0.6-0.77). Conclusion: When trained on a dataset of over 60 thousand breasts, a volumetric U-Net performs as well as expert radiologists at segmenting malignant breast lesions in MRI.
Automated detection and segmentation of non-mass enhancing breast tumors with dynamic contrast-enhanced magnetic resonance imaging
Sep 26, 2018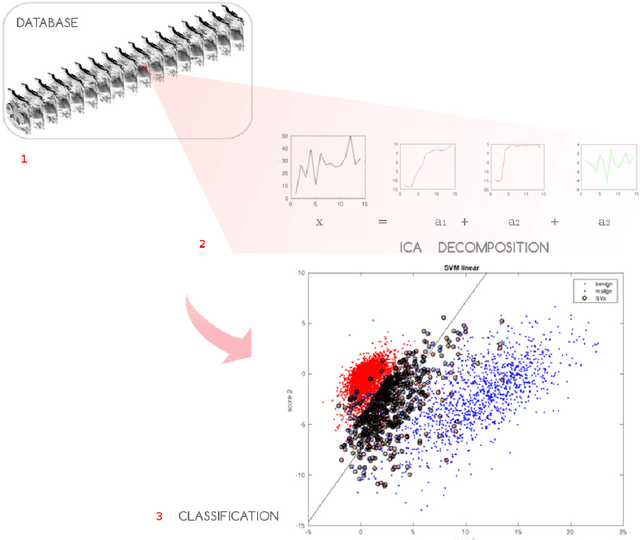
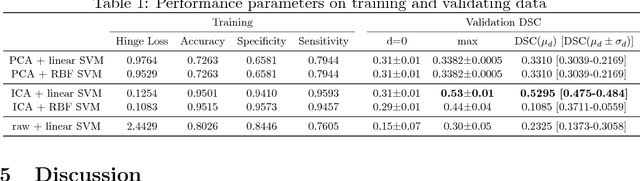
Abstract:Non-mass enhancing lesions (NME) constitute a diagnostic challenge in dynamic contrast enhanced magnetic resonance imaging (DCE-MRI) of the breast. Computer Aided Diagnosis (CAD) systems provide physicians with advanced tools for analysis, assessment and evaluation that have a significant impact on the diagnostic performance. Here, we propose a new approach to address the challenge of NME detection and segmentation, taking advantage of independent component analysis (ICA) to extract data-driven dynamic lesion characterizations. A set of independent sources was obtained from DCE-MRI dataset of breast patients, and the dynamic behavior of the different tissues was described by multiple dynamic curves, together with a set of eigenimages describing the scores for each voxel. A new test image is projected onto the independent source space using the unmixing matrix, and each voxel is classified by a support vector machine (SVM) that has already been trained with manually delineated data. A solution to the high false positive rate problem is proposed by controlling the SVM hyperplane location, outperforming previously published approaches.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge