Jens Meier
Diagnostic Quality Assessment for Low-Dimensional ECG Representations
Oct 01, 2022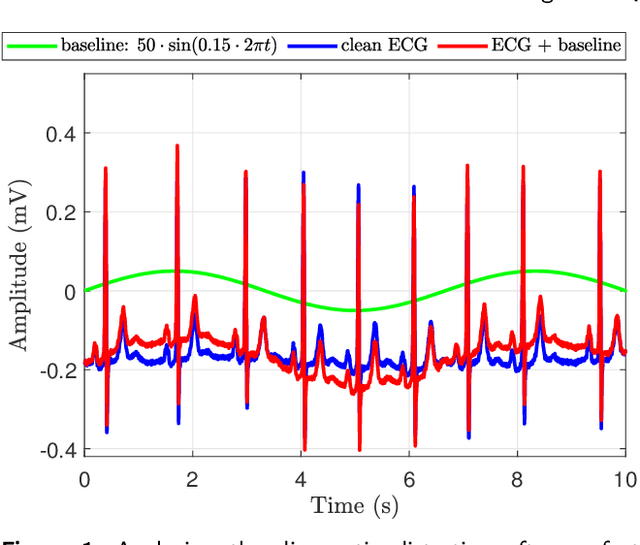


Abstract:There have been several attempts to quantify the diagnostic distortion caused by algorithms that perform low-dimensional electrocardiogram (ECG) representation. However, there is no universally accepted quantitative measure that allows the diagnostic distortion arising from denoising, compression, and ECG beat representation algorithms to be determined. Hence, the main objective of this work was to develop a framework to enable biomedical engineers to efficiently and reliably assess diagnostic distortion resulting from ECG processing algorithms. We propose a semiautomatic framework for quantifying the diagnostic resemblance between original and denoised/reconstructed ECGs. Evaluation of the ECG must be done manually, but is kept simple and does not require medical training. In a case study, we quantified the agreement between raw and reconstructed (denoised) ECG recordings by means of kappa-based statistical tests. The proposed methodology takes into account that the observers may agree by chance alone. Consequently, for the case study, our statistical analysis reports the "true", beyond-chance agreement in contrast to other, less robust measures, such as simple percent agreement calculations. Our framework allows efficient assessment of clinically important diagnostic distortion, a potential side effect of ECG (pre-)processing algorithms. Accurate quantification of a possible diagnostic loss is critical to any subsequent ECG signal analysis, for instance, the detection of ischemic ST episodes in long-term ECG recordings.
ECG Beat Representation and Delineation by means of Variable Projection
Sep 27, 2021


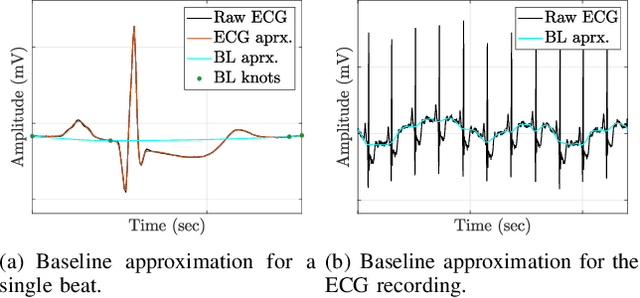
Abstract:The electrocardiogram (ECG) follows a characteristic shape, which has led to the development of several mathematical models for extracting clinically important information. Our main objective is to resolve limitations of previous approaches, that means to simultaneously cope with various noise sources, perform exact beat segmentation, and to retain diagnostically important morphological information. Methods: We therefore propose a model that is based on Hermite and sigmoid functions combined with piecewise polynomial interpolation for exact segmentation and low-dimensional representation of individual ECG beat segments. Hermite and sigmoidal functions enable reliable extraction of important ECG waveform information while the piecewise polynomial interpolation captures noisy signal features like the BLW. For that we use variable projection, which allows the separation of linear and nonlinear morphological variations of the according ECG waveforms. The resulting ECG model simultaneously performs BLW cancellation, beat segmentation, and low-dimensional waveform representation. Results: We demonstrate its BLW denoising and segmentation performance in two experiments, using synthetic and real data (Physionet QT database). Compared to state-of-the-art algorithms, the experiments showed less diagnostic distortion in case of denoising and a more robust delineation for the P and T wave. Conclusion: This work suggests a novel concept for ECG beat representation, easily adaptable to other biomedical signals with similar shape characteristics, such as blood pressure and evoked potentials. Significance: Our method is able to capture linear and nonlinear wave shape changes. Therefore, it provides a novel methodology to understand the origin of morphological variations caused, for instance, by respiration, medication, and abnormalities.
* Codes, experiments, demonstrations are available at: https://codeocean.com/capsule/2038948/tree/v1
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge