Irvin Ho
Prediction of MET Overexpression in Non-Small Cell Lung Adenocarcinomas from Hematoxylin and Eosin Images
Oct 12, 2023Abstract:MET protein overexpression is a targetable event in non-small cell lung cancer (NSCLC) and is the subject of active drug development. Challenges in identifying patients for these therapies include lack of access to validated testing, such as standardized immunohistochemistry (IHC) assessment, and consumption of valuable tissue for a single gene/protein assay. Development of pre-screening algorithms using routinely available digitized hematoxylin and eosin (H&E)-stained slides to predict MET overexpression could promote testing for those who will benefit most. While assessment of MET expression using IHC is currently not routinely performed in NSCLC, next-generation sequencing is common and in some cases includes RNA expression panel testing. In this work, we leveraged a large database of matched H&E slides and RNA expression data to train a weakly supervised model to predict MET RNA overexpression directly from H&E images. This model was evaluated on an independent holdout test set of 300 over-expressed and 289 normal patients, demonstrating an ROC-AUC of 0.70 (95th percentile interval: 0.66 - 0.74) with stable performance characteristics across different patient clinical variables and robust to synthetic noise on the test set. These results suggest that H&E-based predictive models could be useful to prioritize patients for confirmatory testing of MET protein or MET gene expression status.
AI-augmented histopathologic review using image analysis to optimize DNA yield and tumor purity from FFPE slides
Apr 07, 2022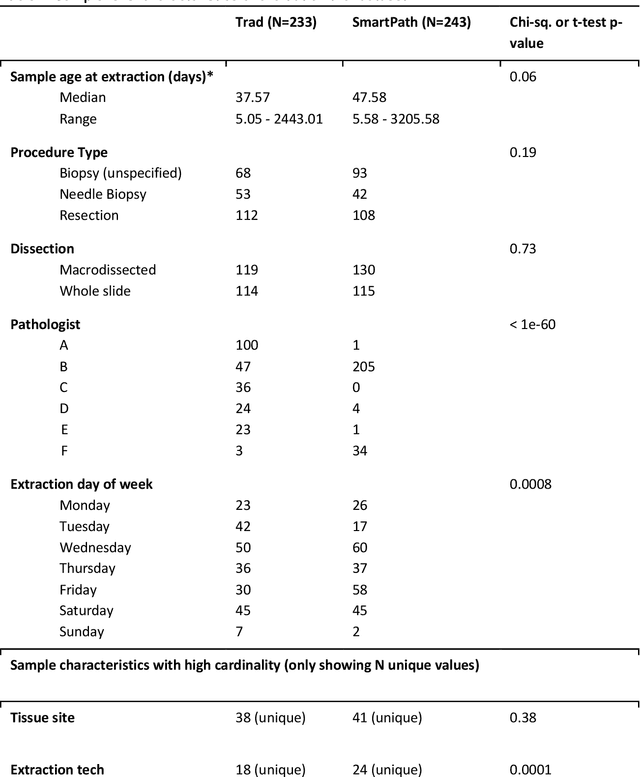

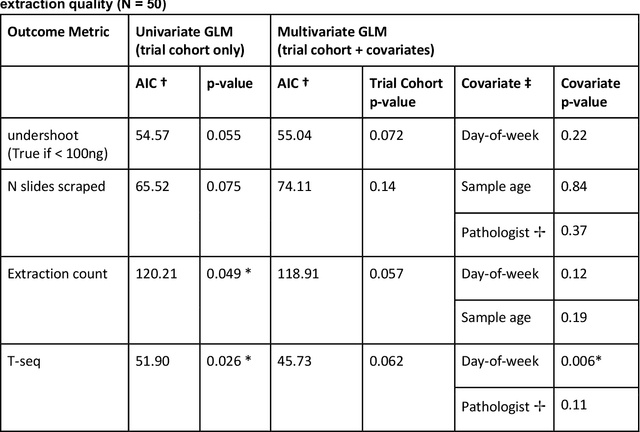

Abstract:To achieve minimum DNA input and tumor purity requirements for next-generation sequencing (NGS), pathologists visually estimate macrodissection and slide count decisions. Misestimation may cause tissue waste and increased laboratory costs. We developed an AI-augmented smart pathology review system (SmartPath) to empower pathologists with quantitative metrics for determining tissue extraction parameters. Using digitized H&E-stained FFPE slides as inputs, SmartPath segments tumors, extracts cell-based features, and suggests macrodissection areas. To predict DNA yield per slide, the extracted features are correlated with known DNA yields. Then, a pathologist-defined target yield divided by the predicted DNA yield/slide gives the number of slides to scrape. Following model development, an internal validation trial was conducted within the Tempus Labs molecular sequencing laboratory. We evaluated our system on 501 clinical colorectal cancer slides, where half received SmartPath-augmented review and half traditional pathologist review. The SmartPath cohort had 25% more DNA yields within a desired target range of 100-2000ng. The SmartPath system recommended fewer slides to scrape for large tissue sections, saving tissue in these cases. Conversely, SmartPath recommended more slides to scrape for samples with scant tissue sections, helping prevent costly re-extraction due to insufficient extraction yield. A statistical analysis was performed to measure the impact of covariates on the results, offering insights on how to improve future applications of SmartPath. Overall, the study demonstrated that AI-augmented histopathologic review using SmartPath could decrease tissue waste, sequencing time, and laboratory costs by optimizing DNA yields and tumor purity.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge